Fertility Hedge Fund? Pros and Cons of Egg Banking
Freezing and storing your own eggs when you are not trying to get pregnant used to be rare. It was something young women with cancer might do, if treatment could badly damage their eggs or ability to ovulate.
Not any more. Oocyte cryopreservation is now promoted as a “hedge” against declining fertility – called by some, anticipated gamete exhaustion (AGE). And if commercial egg freezing isn’t already a billion-dollar industry in the US alone, it might not be long till it is. Egg freezing is being heavily marketed, nudging women’s fears and aiming to make it trendy with slogans like, “Smart Women Freeze”.
Freezing eggs was never going to be as simple as freezing sperm, especially as there is so much liquid (cytoplasm) inside an oocyte. Although the first baby born using a frozen oocyte was in 1986, success rates for IVF with frozen eggs couldn’t compare to fresh eggs or frozen embryos.
It took 2 developments in the 2000s for oocyte cryopreservation to become widely accepted by reproductive specialists: a fast-freezing method called vitrification and an IVF method, ICSI (intracytoplasmic sperm injection) [ASRM]. Vitrification had less impact on embryo development and was much more effective than previous slow freeze methods. And ICSI is thought to improve chances of fertilization, because sperm are injected directly into the cytoplasm. That might reduce the impact of changes in the oocyte’s outer membrane caused by freezing.
The egg freezing process is basically half an IVF cycle. There will be a couple of weeks of ovarian stimulation – hormone injections to get the ovaries to ripen a bunch of oocytes. Alongside, there is monitoring for adverse effects of these fertility drugs, and to determine the timing of the next step.
That next step is more hormones to trigger ovulation. Then comes oocyte retrieval: eggs are picked up with a needle through the vaginal wall into the ovary.
The costs of this could be from $12,000 to over $20,000, including medications and annual storage costs – more, if it takes more than 2 cycles or there are medical complications. If eggs are later used, there will be further costs for one or more ICSI cycles, along with more rounds of hormones and procedures.
In 2012, the American Society for Reproductive Medicine (ASRM) issued a statement that egg freezing was no longer experimental [PDF]. Pre-emptive egg freezing “just in case” was already driving an increase in partial IVF cycles by then. It’s not the only reason for freezing eggs, though. It’s an alternative to freezing embryos for some women who are currently trying to get pregnant, too. The latest available figures from the CDC for the U.S. go up to 2014.
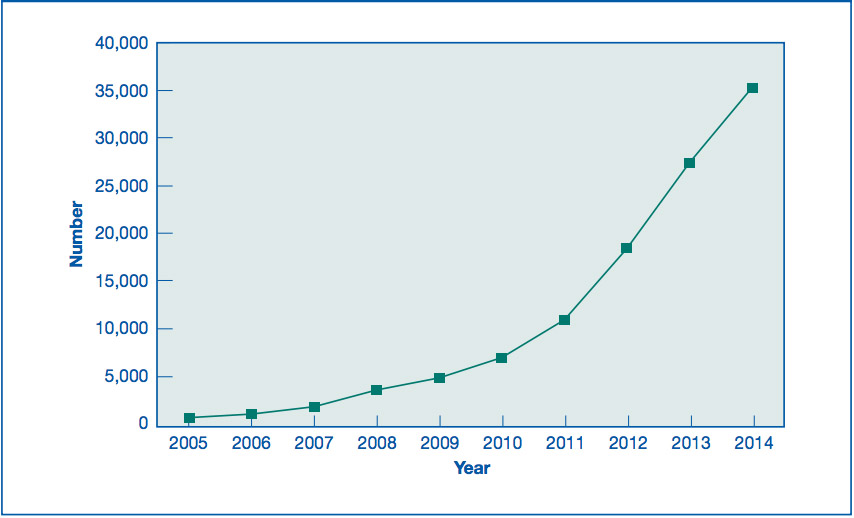
In 2013, debate began about law firms offering the cost of egg freezing as an employee benefit. 2013 was also the year that Sarah Elizabeth Richards’ book on egg freezing was published amid a blaze of promotional publicity, including her piece in the Wall Street Journal, “Why I froze my eggs (and you should too)”.
Since then, there have been high profile announcements by Facebook (in 2014) and Apple (in 2015) that $20,000 a year towards covering the cost of egg retrieval and freezing was now an employee benefit. The U.S. defense forces included it as a benefit for active duty servicewomen in 2015.
Richards reported that she spent nearly $50,000 on egg retrieval and freezing over 2 years – between 36 and 38 years old. The age women are doing this is getting lower, though. Egg freezing is now being marketed to women in their 20s, and you don’t have to look far to find people arguing it should be discussed with all women in their early 30s if they don’t already have a child (example). That’s a no-lose business proposition for reproductive services.
But the American College of Obstetricians and Gynecologists (ACOG) has issued a statement saying that there was not enough data to recommend egg freezing “for the sole purpose of circumventing reproductive aging in healthy women”.
There is a core problem here. The younger women are when they freeze eggs, the less likely it is that they will ever want to use them. They will be infertility patients without ever being infertile. The older women are, the less likely it is that their frozen eggs would make a difference.
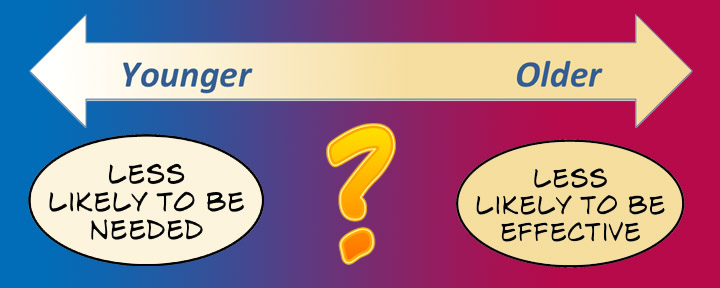
The ASRM has said there is a “relatively high likelihood” that women who freeze eggs before the age of 35 will never use them. Women’s fertility declines gradually from 20 to 30 years of age, then more steeply somewhere around 35, and ever more steeply from about 38, before ending at menopause (on average, around 50) [PDF].
The rate of infertility is over 60% between 40 and 44, but the rate of infertility is still only 30% between 35 and 39 [ASRM]. Pregnancies over 40 carry higher all-round risks, even for the healthiest, fittest women. And by 45, it’s very unlikely a woman can have a baby – with or without IVF.
Of the women who had a baby over the age of 40 in 2011, only about 6% got pregnant using assisted reproductive technology (ART – any IVF technique) [PDF]. (I didn’t find data on how often pregnant women over 40 were having their first baby, and planning to.) For the women who use ART, most will use their own, “real-time” eggs. At 39 years old, about 10% will use donor eggs, rising to 50% of women around 44 [CDC data].
Let’s step through how that might change if a woman whose fertility isn’t immediately threatened decides she would like to freeze eggs in her 20s or 30s for her own use. This is when we should start calculating the odds of being more or less likely to have a baby: life choices, health, emotional wellbeing, and chances of being a mother (and how) could all be changed after taking the first step down this road.
1. Consultation and testing
There will be history-taking, blood, and ultrasound tests. This is to see if (a) there are any conditions that increase the risks of ovarian stimulation, and (b) to try to estimate ovarian reserve (whether there are enough oocytes that could be ripened). For (b), the evidence for the value of these tests is not strong at all, although there is plenty of marketing hype around them. There could be genetic testing, too.
I didn’t find good data on how many women don’t move past this first base, either because they are not accepted as candidates or they change their minds.
This step is a no-lose business proposition for a service provider. From a consumer choice point of view, though, it is fraught with complexity. The bases for the choice that a future self would make in choosing an IVF service provider might be very different from the choice of an egg-freezing option. Years later, another clinic may be having far better outcomes. Yet, the choice of oocyte cryopreservation provider might pre-empt or constrain those later choices.
2. Fertility drugs for ovarian stimulation and ovulation induction (ripening and releasing eggs)
This step involves daily hormone injections for a couple of weeks and frequent monitoring, including vaginal ultrasound.
Like all major hormone treatments, adverse effects are common. A recent systematic review of hormones for triggering ovulation estimated the rate of adverse effects at about a third of women. That includes injection issues and effects of hormones on the body and emotions.
The most serious risk is ovarian hyperstimulation syndrome (OHSS). The review said that moderate to severe OHSS is expected in 3 – 10% of ART cycles. The risk of OHSS is higher for young women. (You can read about OHSS symptoms here.) OHSS can be fatal, so women with serious symptoms will be hospitalized.
The rate of canceling a cycle because of adverse effects, or the cycle not resulting in enough eggs, varies a lot. Anywhere from 2 – 30% of women on ART cycles have been estimated to cancel the cycle or have a low success rate at this point. In the CDC’s national data for 2014, about 10% of cycles were canceled. A study on one clinic’s results for women starting cycles for egg storage reported a 21% rate of too few eggs – and most of those women still had too few eggs after multiple cycles. (The women in that study were all over 34.)
There are big question marks here, because most of the data about ART comes from women who are trying to get pregnant. That might mean a greater willingness to tolerate adverse effects and plough on than a young woman for whom this is not a last chance.
After so many years of IVF, you would think we would have conclusive data on all the key long-term health questions for women and children, but we don’t.
There is more longer term data for women, but it is in mostly older, infertile women having infertility treatment, not young, healthy, fertile women. The data suggest ovarian stimulation doesn’t increase the risk of breast cancer (see here and here), although there is not enough data to put other reproductive cancers in the clear for fertile women using fertility drugs (here).
There are still open questions about the longterm health of people born after IVF (here, here and here). A systematic review in 2009 found no longterm health data in children or adults born after oocyte freezing, and non-systematic reviews in 2013 and 2014 didn’t either.
3. Oocyte retrieval (egg pick-up)
The success rates for this aren’t separated from the data in the previous step. The common issues here are pain during, and for 1 of 2 days after, the procedure, plus adverse effects of the anesthesia/analgesia.
The less common adverse effects are bleeding, damage to organs, pelvic infection, and serious anesthetic complications (from less than 1% to 2 – 3% per type of complication).
4. Repeat cycles
I didn’t find data on how many cycles women have, or how many eggs women store.
Around 90% of eggs will survive the freezing/thawing process in the short-term at least [ASRM], but those won’t all successfully fertilize. And only a minority of embryos lead to a live birth [CDC]. So 1 egg is a very long way from 1 baby.
There isn’t very strong data on how many eggs to store. Rosalie Cabry and colleagues refer to studies suggesting at least 12 are needed for 1 pregnancy, with others suggesting 22 for women 37 years old or younger and 55 for women over 37. On average, Cabry wrote, that means 2 – 3 cycles per hoped-for birth.
5. How likely are the eggs to be used?
It’s unlikely women will use their frozen eggs (unless perhaps they are donated/sold to others), but I didn’t find much data to narrow this down. In 1 study, where 505 women had frozen eggs across a 5-year period, only 20 had come back to try for a pregnancy at that point (4%). Kevin Doody from the Society for Assisted Reproductive Technology (SART), which maintains a national registry of most ART data, reported to Time magazine that there were 176 babies born after egg freezing in 2012 and 2013 in the U.S. nationwide. (There are tens of thousands egg freezing cycles each year.)
The majority of women who set out down the egg freezing path are likely to have some eggs. But most of them – and almost all younger women – may never have infertility problems, either because they will get pregnant without medical help, or they let go of the idea of pregnancy.
6. The chances of having a live birth because of stored eggs
Here the issue isn’t the success rates for stored eggs: it’s whether the success rates are higher with stored eggs than starting from scratch.
ART data is complicated. The numbers – for example at a single clinic – can be too small to be a reliable gauge of what might happen to others. There is often no good comparative data. And apparent success can be exaggerated by measures like embryo transfers or clinical pregnancy, not live birth. The miscarriage rate, including of the earliest chemically detectable pregnancies, can make quite a bit of difference, especially in older women. Both early and late pregnancy loss can be devastating, and that has to be taken into account.
What you select as the denominator makes a big difference. For example, the later down the line you start the clock ticking – say, only after eggs are already frozen – the more unsuccessful outcomes you leave out from the calculations. And that inflates the success rate. That’s called selection bias. This hypothetical example shows selection bias in action:
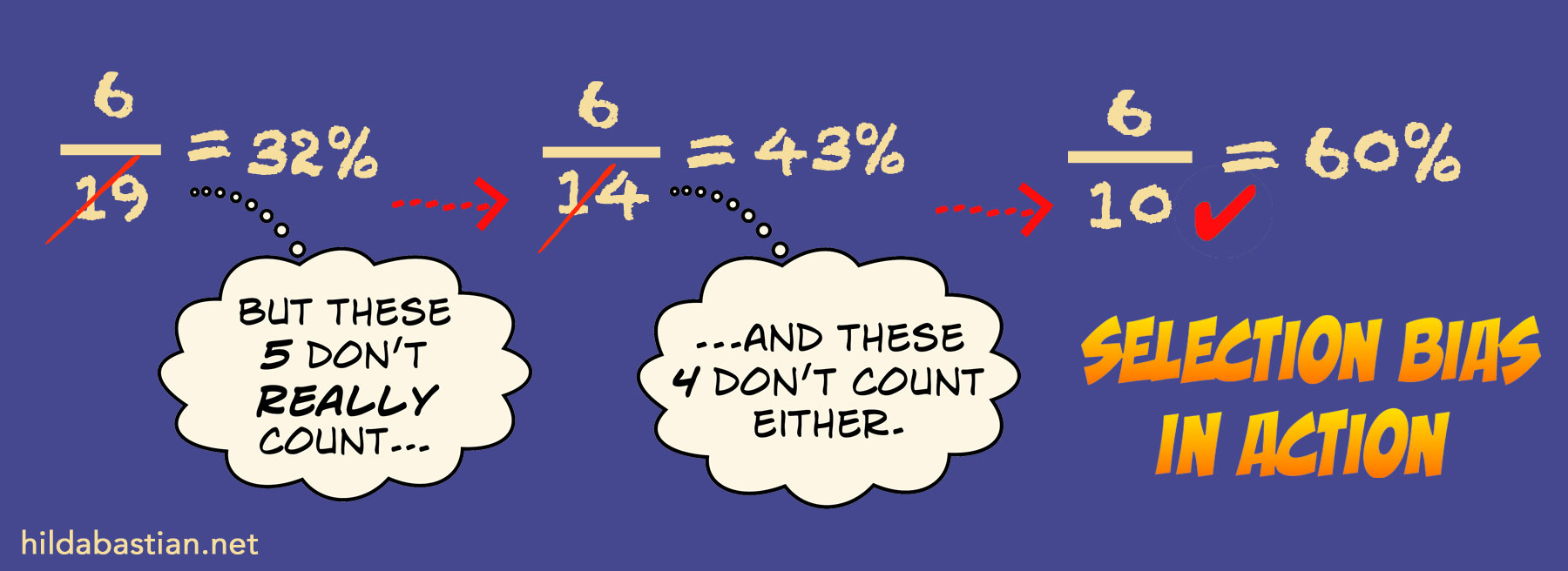
For women who start an ART cycle with their own “real-time” egg(s), the live birth rate is about 35% in the mid-30s, dropping to 16% at 40 years of age [CDC data]. With embryos from donor eggs from younger, highly selected egg donors (usually in their 20s or early 30s), the rate is close to 45% even at 40 years.
But there don’t seem to be any direct comparison studies with a woman’s younger self being her donor, so we can’t be certain at all how using stored eggs stacks up. The rates reported so far with autologous oocyte cryopreservation (freezing your own eggs) are closer to using “real-time” eggs than donor eggs from selected young donors. Those SART figures reported to Time showed a 23% live birth rate – but we don’t know anything about age (of oocytes or the women) or whether vitrification was used, so it’s not very helpful. It’s not encouraging, though.
Aylin Pelin Cil and colleagues pooled data from 10 studies with 1,805 women. Using a statistical model, they estimated live birth rates of around 20 – 30% in women below 30 and half that in older women. Again, no higher than ordinary ART. They suggested 36 as a cut-off age for egg freezing.
Neelam Potdar and colleagues take issue with some aspects of Cil’s modelling. They analyzed 17 studies, but decided live birth rates couldn’t be estimated. Potdar concluded the rate of pregnancies lasting longer than 20 weeks was around 7% per thawed egg – and somewhat lower for women using their own eggs.
The success rates represent enormous joy. The high rate of pregnancy loss only hints at the emotional burden. Pamela Mahoney Tsigdinos writes in “The sobering facts about egg freezing that nobody’s talking about” in Wired:
We’ve…been led to believe that science has mastered Mother Nature. This is not true. I know. I am a former patient of three clinics in the Bay area, all of which were happy to sell me services as long as I could pay the bill. I had multiple fresh and frozen embryo transfers. Instead of taking home a baby, I came away with tremendous heartache…
The emotional toll associated with family-building failure can be crushing. The scientific fascination with the latest protocol and the marketing of fertility procedures as a lifestyle enhancer the past few decades has unwittingly led to a disregard for the emotional responses of these medical procedures, which creates a different kind of health concern – one involving mental health.
With the medical uncertainties about egg freezing, comes the uncertainty about whether having “banked” eggs changes women’s life decisions.
Some are encouraging egg freezing as a way to lessen anxiety about loud biological clock ticking. It’s not the only option for coping with anxiety or regrets, though. And we can’t yet rule out that a sense of reassurance because of the backup plan means some women delay childbearing longer than they would have – increasing the chances of exactly the outcome they are worried about.
People talk about “egg banking” as though it’s insurance. I think both “banking” and “insurance” are misleading ways to look at this. This language gives an impression of more security than freezing eggs can deliver. And it doesn’t convey the health and emotional risks.
Egg freezing is important for women whose fertility is threatened by something other than age. It’s hard for me to see it as a step forward for others, though. There is no strong data showing important benefit, set against high physical, emotional, and financial costs. Even knowing that, it will still be seen as worthwhile to some women, of course. But it’s arguable how well-informed women are at this point. The promotional hype and service provider advocacy look more exploitative than empowering to me.
~~~~
Further reading on non-medical issues around egg freezing:
Social, political, class, ethical, legal, and commodification aspects of egg banking: Francis Baylis, Lisa Ikemoto, Heidi Mertes (2013), Heidi Mertes (2015), John Robertson.
Studies on women’s views of egg banking: Marije de Groot et al, rooke Hodes-Wertz et al, Dominic Stoop et al.
The cartoon and illustrations are my own (CC-NC-ND-SA license). (More cartoons at Statistically Funny and on Tumblr.)
* The thoughts Hilda Bastian expresses here at Absolutely Maybe are personal, and do not necessarily reflect the views of the National Institutes of Health or the U.S. Department of Health and Human Services.


I have been the principal investigator in the largest amount of research on women’s feelings about egg freezing to date and have found that it also relives tension and empowers women
Women are capable of reading the scientific literature and making their own decisions on whether or not this technology is beneficial to them. Frankly, I find the push back (both here and other in other places) on this particular procedure rather insulting and patriarchal. Arguably a 38 year old woman and a woman about to undergo chemotherapy are in a similar situation – 5 years from now neither one is likely to be able to conceive on her own. If it’s useful for the woman undergoing chemo, I fail to see why it is not useful to the 38 year old. Certainly the success rate is not 100%, but for some a 20% chance is better than a 0%. Women are capable of reading the literature and making that choice for themselves. They don’t need other people to “protect” them. They are not illiterate children.
While many women would certainly be capable of reading the scientific literature, they wouldn’t have access to a lot of it if they weren’t academics, as it’s behind paywalls. And a lot of it is far more negative than the post I wrote, so access to that wouldn’t avoid critical writing on the subject. If a woman can cope with all that, she can certainly cope with this post.
What most women do have easy access to, is a lot of marketing material with very biased data and information – leading to impressions that the success rate at older ages is as high as you mention, or even higher, when the data doesn’t support that at all.
There’s a very big difference between a woman with cancer in her 20s or early 30s and freezing eggs, and being in your late 30s. Those years make an enormous difference. For a 43-year-old, chances are going to be better with donor eggs from someone younger than 38: and it’s a very long way from 0%, with or without assisted reproduction. So the situation is not analogous at all.