This month, Arcturus’ self-amplifying mRNA vaccine was approved for use in the UK. There were also new clinical trial results for another…
New Vaccine Hopes, Adverse Reactions, & a Developer Clashing With Regulators: A Month of Highs & Lows

We’ve well and truly reached cherry-picking season on Covid vaccine data! It’s definitely easier to fall into the trap of claims based on a biased selection of information – and harder to keep up with developments. With over 1 billion doses of Covid vaccines injected globally, the amount of datasets and studies is vast.
Fortunately, there are now well over half a million people in phase 3 trials for Covid vaccines, providing an important base of critical information. That number could get close to a million in the coming months. A big thank you to everyone participating in the trials that made this possible.
Because there’s so much to unpack in this post, lengthy sections start with a summary of key points at the top – and there’s a contents list with links so you can navigate around. But first some explanations for heavily used terminology in this post.
Key terms used in this post:
- Vaccine efficacy is a rate of risk reduction in symptomatic Covid-19 unless otherwise specified. Vaccine efficacy of 80% or 90% means if a vaccinated person is exposed to the virus, their risk of getting the disease is lowered by that proportion, so it depends on how high their risk of being exposed, and that varies. It’s not an absolute drop in percentage points. (Efficacy is for results from phase 3 clinical trials; effectiveness studies follow that.)
- When available, a range of statistical certainty for efficacy is shown, eg 92% (CI: 88-95). The distance between 88% and 95% in this example is small: it means there is a lot of certainty that 92% is about what we can expect. However, the wider that range is, the more uncertain we are.
- The rate of efficacy set for whether a Covid vaccine works well enough is 50% (with a CI starting at 30% at least).
- And I have a post explaining the terms used, and assessment processes, for adverse events and safety in these trials – including the difference between “severe” and “serious”.
Contents
- Major turning points and new hopes
- Community impact studies
- Trial results in children and teenagers
- Study results in people with compromised immune systems
- Vaccine combination trials
- The landscape: 11 vaccines with phase 3 results
- What else is new for the 11 vaccines
- Overview of phase 3 trials and what’s new
- Addendum: phase 3 trial recruitment status and studies
- Sources for study records and notes on using the collection
Major turning points and new hopes
I think three major recent developments stand out, but could be overlooked amidst all the massive activity and vaccine dramas:
- End of the Covid vaccine need for super-freezers is in sight;
- The first all-coronavirus vaccine entered clinical trial; and
- More globally accessible powerful vaccines are coming into view.
All those claims that a vaccine has the advantage of not needing super-freezers really should stop. It turns out, only 2 ever will have needed it – BNT-Pfizer and Moderna vaccines – and both of them now have second generation versions that don’t need them in trials. Fingers crossed that demanding and expensive era will soon be over!
Next up, with the concern about variants and the possibility of a SARS-CoV-3 or a MERS-2, an all-coronavirus vaccine could be critical. And one has recently started a first-in-human trial in the US. It’s a protein subunit vaccine with an adjuvant to boost it, and it’s being developed by a US Army lab – the Walter Reid Army Research Institute. Countering future variants with a universal coronavirus vaccine is the ambition: “We are in this for the long haul”. (Records for this vaccine in my collection here.)
Finally, there’s another generation of vaccines that’s much closer, but are shaping up to be key to very widely accessible vaccines. Non-profit protein subunit vaccines from Cuba are in advanced phase 3 trials and likely to supply many countries if successful, and Vietnam’s first locally-developed protein subunit vaccine is nearing phase 3 trial as well (more on these below).
Another potentially critical option took a big step recently, too. It’s the saRNA (self-amplifying RNA) vaccine from Imperial College in London, a publicly funded development effort which aims to sidestep the drug industry and forego all licensing fees as well as profits. In a preclinical study, it provided more immunity than the AstraZeneca vaccine.
This group is going down the human challenge trial road. The first volunteers in their early tests to establish human challenge methods have already left quarantine. They are now running a 90-person study to determine the minimum amount of virus needed to infect someone. (Records on this vaccine in my collection are here.)
A third major development is driven by public interest coupled with cutting edge technology. As Carl Zimmer reports in a must-read article in the New York Times,
A new vaccine for Covid-19 that is entering clinical trials in Brazil, Mexico, Thailand and Vietnam could change how the world fights the pandemic. The vaccine, called NDV-HXP-S, is the first in clinical trials to use a new molecular design that is widely expected to create more potent antibodies than the current generation of vaccines. And the new vaccine could be far easier to make.
It couples traditional, widely accessible technology – the vaccine can be mass-produced in eggs the way flu vaccines are – with a major advance in the way the virus spike is modified. One of the advantages that the BNT-Pfizer, Modern, and J&J vaccines have is using a protein spike with 2 prolines (“2P”). This is beautifully explained in Zimmer’s article – and below is my cartoon take!
The prolines stabilize the spike, which can change shape, leading to the production of antibodies to the changed protein. Though some developers argue that spikes are better as they are, the 2P spike has a lot going for it. It’s the brainchild of Jason McLellan and colleagues – and he and his team went on to develop one with 6 prolines, called Hexapro (that’s the HXP in the vaccine’s name).

And Hexapro can be licensed by low and middle income countries without royalties. It’s coupled with another development that’s non-profit – the Mount Sinai-developed Newcastle Disease Virus platform (that’s the NDV in the name).
Even though they have been slower to develop than others, these vaccines could reach people’s arms faster in many countries because of the huge discrepancy in global access to vaccines in 2021. The situation has been made even more intensely critical than we had expected with the major shortfall between the original estimates for production of the Oxford-AstraZeneca vaccine and the concentration of vaccine manufacture in India. With domestic needs not covered by original plans and a massive coronavirus wave sweeping through the country, the exports so much of the world was counting on haven’t been possible.
Madhukar Pai and Manu Prakash underscore this in a Washington Post op-ed, writing that the Indian wave shows we’re now making “our biggest strategic mistake”. In the nearly unvaccinated continent of Africa, they point out for example, there are 10 countries which zero ventilators and only about 2,000 ventilators spread across almost all the rest. With global deaths from Covid-19 soaring over 3 million now, when the studies come showing the cost of the global rich-countries-first approach, it’s going to be a brutal reckoning.

Community impact studies
- Several controlled community studies confirm the high effectiveness of BNT-Pfizer vaccine against symptomatic Covid-19, with reduced but still high effectiveness against several variants of concern, and evidence of high levels of reduction of hospitalization and transmission of the coronavirus.
- Some controlled evidence confirms the community effectiveness of the Moderna and J&J vaccines against symptomatic Covid-19, including where infection caused by variants of concern is common.
- There is some evidence that the effectiveness of Sinovac’s CoronaVac offered protection against Covid-19 and hospitalization in an outbreak of a major variant of concern, although it was reduced.
- There is some evidence from Israel that high rates of vaccination with BNT-Pfizer vaccine in age groups with high interactions with others reduces infections in unvaccinated people in their communities, including under-16s; and from the UK suggesting vaccination of healthcare workers with BNT-Pfizer or AstraZeneca vaccines reduced the risk of infection of members of their household.
- Some early community data from the UK suggests the Oxford-AstraZeneca vaccine, with second dose delayed, might increase in effectiveness over a month to 70% or more (as it did in exploratory trial analyses here and here).
Once you start studying vaccine rollouts, you have to struggle with a lot of “noise” in what’s sometimes called “real world data”: you often can’t even being to untangle the effects of differences in who gets vaccinated first, for example, or the effects of community-wide actions like lockdowns. (I discussed that a bit more when I introduced this segment last time.)
So I concentrate on community impact studies that have used a design that reduces the biases (such as matched controls for effectiveness studies), and done in very broad groups of people or geographic regions. I tag these studies in my records collection. The goal here is to maintain a rough picture of what we know that either confirms (or not) that the results of the randomized trials apply to the broad community; or that addresses questions the trials could not.
I’ve added 8 new studies, bringing the total featured across my posts – and summarized above – to 12. There are 10 studying BNT-Pfizer, 2 each for AstraZeneca and Moderna, and 1 each on the J&J vax and CoronaVac. (An explanation of my search strategy for studies is at the end of this section. And I look at studies on immunosuppressed people separately below.)
But before we get to the new featured group and an update on “whole-of-town” studies, here are some other studies with less-controlled designs that look at vaccines with less other evidence, performance against variants, and transmission.
BNT-Pfizer vaccine:
- The SIREN study in the UK involved over 23,000 healthcare workers who got tested for SARS-CoV-2 fortnightly, and antibodies monthly. The researchers estimated that a week after their second dose of BNT-Pfizer vaccine the rate of protection against both symptomatic and asymptomatic Covid-19 was 85% (CI 74-96).
- From Israel, a study found that people who got infected after being vaccinated had lower viral loads from 12 days after the first BNT-Pfizer shot.
- The rate of death from Covid-19 was more than halved among residents of nursing homes after vaccination with the BNT-Pfizer vaccine – from a large study in the US.
AstraZeneca (AZ) vaccine:
- A prospective observational study of self-reported adverse events in the UK provided the first data we’ve seen in a group with many older people for the AZ vaccine. Adverse events were still substantially more common than for BNT-Pfizer vaccine, suggesting this vaccine will stay at the higher end of the Covid vaccine spectrum for adverse reactions.
Sputnik V vaccine:
- A study in San Marino suggests that the rate of adverse reactions to the Sputnik V is at the high end of the spectrum for Covid vaccines. (Preprint) (More on this.)
Sinovac’s CoronaVac:
- In Chile, comparison data from the National Health Fund was adjusted for age, gender, region, income, and nationality. A single dose of CoronaVac wasn’t protective, but from 14 days after the second dose, it was estimated to be 67% effective against symptomatic illness (CI 65-69), 85% against hospitalization, 89% against intensive care admission, and 80% against death (CI 73-86). (Government data report)
- Brazil vaccinated around 95% of people aged 80 and over, 77% with CoronaVac (the rest with AZ), and as their vaccination rate soared, their Covid mortality halved (in a context of a P1 (“Brazil”) variant outbreak). (Preprint)
- Considerable uncertainty around this, but this comparison suggested that 2 doses of CoronaVac was around 50% effective after 2 weeks in healthcare workers from the University of São Paulo, rising to around 70% after a month. And most of the illness in that outbreak was caused by the P1 (“Brazil”) variant of concern. (Preprint)
Now to this month’s more rigorous studies. This month’s featured group includes 8 new controlled studies and there’s an update on “whole-of-town” studies. (The WHO’s evidence assessment of Sinopharm’s Beijing vaccine had a slide of results from a test-negative case-control study in Bahrain, but I couldn’t find any more detail, so I haven’t included it.)
- Brazil – Sinovac’s CoronaVac, healthcare workers in Manaus, and the P1 variant
- Israel – Some breakthrough possible after BNT-Pfizer with variants, especially for the partially vaccinated
- Qatar – Variants of concern reduced the effectiveness of BNT-Pfizer vaccination, but protection was still high (and very high for severe disease)
- Israel – As 16-50 year-olds vaccinated quickly with BNT-Pfizer, the rate of infection in the under 16s around them dropped in sync
- UK – Vaccination with BNT-Pfizer (mostly 2 doses) led to a major reduction in infection and hospitalization for Covid-19 in 80-83 year-olds
- USA – Early study data confirms the effectiveness of the J&J vaccine
- USA – The mRNA vaccines were highly effective against both symptomatic/asymptomatic infection in an outbreak with mostly variants
- UK – Unvaccinated household members of someone who has tested positive for the coronavirus may be less likely to get infected themselves if the infected person had been vaccinated
- Update on “whole-of-town” studies
Brazil – Sinovac’s CoronaVac, healthcare workers in Manaus, and the P1 variant
Manaus, the largest town in the Amazon, Brazil, experienced a major outbreak dominated by the local variant of concern, P1. It was estimated to be causing around 75% of Covid-19 infections at the time of this study. There were 53,153 healthcare workers living in the town at the time (as well as over 14,000 who lived elsewhere). There wasn’t a specific Covid testing service that they all used.
This preprint reports the first of a series of planned analyses based on linking data from a range of registries. It covered just over 2 months from January 12. For healthcare workers who tested positive, they matched another who tested negative up to 3 days earlier or later, was in the same age group, and lived in the same neighborhood in Manaus. They were able to establish 393 case-control pairs with symptoms, and another 135 pairs who had no symptoms.
Effectiveness of a single dose of Sinovac’s CoronaVac was:
- For symptomatic Covid-19 from 14 days after the dose: 49.6% (CI 11–71); and
- For Covid-19 with or without symptoms, from 14 days after the dose: 35.1% (CI -7 to 61).
There weren’t enough people who had 2 doses at that point for a reliable estimate. There were only 5 samples genotyped for variant, so they can’t say how many of these healthcare workers had the P1 variant – but 4 of those samples were P1, which is consistent with the overall level in the town. So this study suggests CoronaVac provided a valuable level of protection against P1.
Test-negative case-control study: Hitchings 2021 (preprint) (study protocol)
Israel – Some breakthrough possible after BNT-Pfizer with variants, especially for the partially vaccinated
This study comes from the Clalit health fund in Israel, which covers 53% of the population. They looked at 2 groups, partially and fully vaccinated people. “Partially” were people who tested positive between 14 days after the first dose of BNT-Pfizer vax and 1 week after the second; “fully” were those who tested positive more than a week after the second. (A reminder: a week after the second dose was the efficacy outcome for the big phase 3 trial.)
For each person who tested positive (“case”), they matched an unvaccinated person with similar test date, age, sex, ethnic sector, and geographic location. They established 149 matched pairs of fully vaccinated people and 247 of partially vaccinated people, with sequencing of the virus that infected them.
There were only 3 categories of virus: B.1.1.7 (“British”), B.1.351 (“SA”), and various types of wild (original) SARS-CoV-2. Around 90% were infected with B.1.1.7, so there were too few of the others to draw firm conclusions about them. It wasn’t a study designed to measure effectiveness. The study’s hypothesis was if the proportion of a variant was higher in the vaccinated than the unvaccinated people, then it meant the vaccine could be less protective against that variant.
They concluded there could be more breakthrough infections from the variants, particularly for partially vaccinated people, but protection from the vaccine remains high – in the context of mass vaccination with both doses, and a lot of public health measures to control coronavirus spread.
Case-control study: Kustin 2021 (preprint)
Qatar – Variants of concern reduced the effectiveness of BNT-Pfizer vaccination, but protection was still high (and very high for severe disease)
While Qatar was scaling up its rollout of the BNT-Pfizer vaccine, a wave of disease caused by the B.1.1.7 (“UK”) variant began in mid-January, and then another caused by the B.1.351 (“SA”) variant began in mid-February.
The researchers used national registries for this study. It’s not explicitly stated, but it sounds like every SARS-CoV-2 infection was being sequenced to identify if a variant caused it. The testing register includes why the test was done – for symptoms, contact tracing, etc. For this study, a “case” was any person with Covid-19 caused by B.1.1.7 or B.1.351, as well as anyone with severe, critical, or fatal disease. They tried to match each to a person testing negative (“control”), by age, sex, nationality, and reason for getting tested. They didn’t use timing of the test for matching, so analyzed the data by timing of the test and by calendar week to try to ensure that had not influenced the results (sensitivity analyses).
Estimated vaccine effectiveness at 14 or more days after the second dose of BNT-Pfizer:
- Against B.1.17 variant: 89.5% (CI 86-92);
- Against B.1.351 variant: 75.0% (CI 71-79);
- Against severe, critical, or fatal disease (predominantly the variants): 97.4% (CI 92-99.5).
Test-negative case-control study – Abu-Raddad 2021
Israel – As 16-50 year-olds vaccinated quickly with BNT-Pfizer, the rate of infection in the under 16s around them dropped in sync
This analysis was in members of another large health fund in Israel, Maccabi. (It covers close to 2 million people.) Vaccination started on December 19, and by January 30, about a third of their members had got at least 1 dose. The vaccine was BNT-Pfizer, and people aged 16 and over were eligible.
They looked at 223 communities with a similar epidemic pattern before the vaccine rollout, with relatively low outbreaks so that rates of infection-acquired immunity were low. They were 80% of the country’s communities, and included 69% of the fund’s members.
The researchers calculated the rate of positive SARS-CoV-2 tests at 3 time intervals, allowing 35 days to consider that vaccination was providing protection. They found that as the rate of vaccination grew from time point to time point, the rate of infection in unvaccinated people around them dropped in proportion – but not in people over 70. People over 70, they presumed, were less likely to be interacting as much with younger groups of people.
As the 16-50 year-old age group were more most likely to be interacting a lot with the under 16s, they looked specifically at that relationship. And there was a strong correlation between adults in that age group getting vaccinated, and the rate of positive tests falling in the under-16s.
Controlled interrupted time series study – Milman 2021 (preprint)
UK – Vaccination with BNT-Pfizer (mostly 2 doses) led to a major reduction in infection and hospitalization for Covid-19 in 80-83 year-olds
When the UK began its rapid vaccine rollout, over 170,000 people aged between 80 and 83 who didn’t live in care homes got a first dose of BNT-Pfizer between December 15 and 20, with a second dose scheduled for 21 days later: 79% got that dose within 26 days. At the same time, the UK was experiencing a wave of infection caused by the B.1.1.7 (“UK”) variant of concern, and went into a national lockdown soon after the vaccination rollout began.
They matched the 80-83 year-olds with individuals aged 76-79, who weren’t yet eligible for vaccination, excluding those who had a previous SARS-CoV-2 infection. Additional criteria for finding the individuals to pair were gender, area of residence, a measure of economic status, ethnic group, health status, living arrangements, flu vaccine since 2020, and recent emergency hospital stays.
They were able to match 131,236 of the 80-83 year-olds (77%), and analyzed vaccine effectiveness:
- Against hospitalization 35 to 41 days after the first dose (which was 7 days after 80% had a second dose): 75.6% (CI 53-88);
- Against infection (same time): 70.1% (CI 55-80).
Matched case-control study – Mason 2021 (preprint)
USA – Early study data confirms the effectiveness of the J&J vaccine
This was a study of 2,195 people in the Mayo Clinic’s health system who were vaccinated with the J&J vaccine. A control group of 10 unvaccinated people for each J&J-vaxed person was assembled, using a propensity score. The “control” group had to come from exactly the same Zip (area) code, and the scoring system was used to find people of similar age, sex, race, ethnicity, and number of previous SARS-CoV-2 tests.
From day 15 after the single shot J&J vax, vaccine effectiveness was 76.7% (CI 30-95) – an outcome consistent with the efficacy rate in the phase 3 trial. This is an ongoing study.
Propensity-matched case-control study – Corchado-Garcia 2021 (preprint)
USA – The mRNA vaccines were highly effective against both symptomatic/asymptomatic infection in an outbreak with mostly variants
This study analyzed the California Department of Health’s SARS-CoV-2 testing service, which includes all Covid diagnostic tests in the state. At the time, about two-thirds of the people testing positive had been infected by one of 3 variants of concern – B.1.1.7 (“UK”), or “California” variants, B.1.427 and B.1.429. Vaccination could be with BNT-Pfizer or Moderna vaccines.
Each day during the study they randomly selected tested individuals with a telephone across the state to invite them to participate (in English or Spanish). For every person who tested positive, they sought to recruit a person who tested negative in the same week as their control, in the same age group, of the same sex and region. They were able to identify 320 “controls”, and 325 “cases” – 26% of whom had no symptoms.
Vaccine effectiveness against symptomatic/asymptomatic Covid-19 was:
- In the first week after 1 dose: 19.7% (CI -126 to 72);
- In the second week after 1 dose: 66.3% (CI -69 to 93);
- In the third week after 1 dose: 58.9% (CI -10 to 85);
- In the first week after 2 doses: 73.8% (CI 15-92);
- In the second week after 2 doses: 78.4% (CI 23-94);
- 15 days+ after 2 doses: 85.7% (CI 67-94).
In this study, they asked unvaccinated people about vaccination intention, and if hesitant, why. Concern about adverse events/safety was the main reason.
Test-negative case-control study – Andrejko 2021 (preprint)
UK – Unvaccinated household members of someone who has tested positive for the coronavirus may be less likely to get infected themselves if the infected person had been vaccinated
A large study of the impact of vaccination on the risk of infection in household contacts in England included a matched case-control study. The researchers were looking at the question of whether unvaccinated people were less likely to get infected if someone in their household tested positive for SARS-CoV-2 after Covid vaccination than household contacts of infected, unvaccinated people. People had a single dose of either BNT-Pfizer or AstraZeneca (AZ) vaccine, and results were measured 3 weeks+ after that.
In this section of their analyses, they matched people on age, sex, region they lived in, the week of the test, household type, and a measure of socioeconomic status. :
- 1,513 household members of AZ-vaccinated people who had tested positive, with household members of unvaccinated people who tested positive; and
- 2,694 household members of BNT-Pfizer vaccinated people who had tested positive, with household members of unvaccinated people who tested positive.
We don’t know from this study how high the risk was that these people got infected somewhere other than home. The researchers measured the odds of a household member getting infected (odds ratio). For both groups, the odds were half or less compared to people sharing households with unvaccinated people with a SARS-CoV-2 infection. After a single dose of the AZ vaccine, it was about 60% (OR 0.62, CI 0.48-0.79); for BNT-Pfizer, it was about 50% (OR 0.51, CI 0.42-0.62).
Taking all their analyses together, the researchers concluded the risk of household transmission is 40-50% lower when a person had a vaccine dose more than 21 days previously, with a similar rate for both vaccines.
Matched case-control study – Harris 2021 (preprint)
Update on “whole-of-town” studies
A star in this category is Project S in Serrana, Brazil. (Background here.) It’s a registered stepped wedge trial for all eligible adults in the town, broken into 4 clusters, with volunteers vaccinated with 2 doses of Sinovac’s CoronaVac with a 4-week interval. Vaccination quarter by quarter was spaced a week apart – so there are a few weeks between the first and the last.
They finished vaccinating the last quarter of the town on April 11. The community was all-in for this study – on the days people could get vaccinated, cars with loudspeakers reminding everyone drove around the streets. In the end, there were 27,711 in the trial, with a stunning 98% getting their second dose (see the trial register record). That’s not far short of their original hoped-for 30,000 participants. It’s about 60% of the residents – but a lot of people weren’t eligible (too young, for example).
Serrana, like other parts of Brazil, was hit with a major Covid wave while this whole-of-town vaccination got underway. We won’t have to wait long to find out what this study will show: the first results are due in May.
There are some other “whole-of-town” (or community) Covid vaccine experiments going on to explore effects on transmission and community immunity:
- 4,000 people in Hutterite communities in Canada being vaccinated with Moderna (“self-isolated” and contained enthnoreligious communities);
- Around 46,000 people in Schwaz, in Austria’s Tirol, which became a variant hotspot for B.1.351 (“SA” variant), were vaccinated with the BNT-Pfizer vaccine in March, in a study that will run for 6 months; and
- A recent new addition: 106,000 people in Botucatu in Brazil will be offered the AZ vaccine – and there will be sequencing to identify which virus infected them for everyone who gets Covid-19. First effect of this planned study? Applications for rental properties in the city are soaring. [Update May 14] 80,000 doses have arrived and the vaccination drive starts on Sunday.
And then there are small nations that vaccinated extraordinarily quickly, which will no doubt be closely studied, including:
- Bhutan, which administered the first shot of AZ/Covishield to 93% of its adults in 2 weeks;
- Gibraltar, which achieved close to total vaccination, mostly with BNT-Pfizer vaccine, after a “most desperate and devastating winter”;
- Navajo Nation, which is vaccinating with the mRNA vaccines; and
- San Marino, which hoped to be fully vaccinated from age 16 up by the end of May, 85% Sputnik V and 15% BNT-Pfizer. Currently they are at around 70% with at least 1 dose, 85% Sputnik V and 15% BNT-Pfizer, with a test positivity rate of 1.2% (that’s low). They have enough vaccine, but are facing an obstacle with the under-40s, which might jeopardize reaching community immunity as there is a lot of interaction with surrounding Italy.
And of course Israel, population 9 million, will continue to pump out results of large-scale BNT-Pfizer vaccination: they have now vaccinated 78% of people aged 16+.
My search strategy for these studies of healthcare outcomes: I search PubMed and EuropePMC (because it has a different search engine to PubMed and covers a range of preprint servers) as well as the Cochrane Covid-19 study register, scan the preprints from bioRxiv and medRxiv that are tagged as Covid-19, and do broad searches in multiple languages and countries in Google News daily for reports. I can also come across studies via Twitter.
A moving act of reconciliation from Blackfoot First Nations in Montana: they donated a surplus of mRNA vaccine doses to Canadians in nearby Edmonton.
Trial results in children and teenagers
There are trials for children and/or teenagers definitely underway or completed, for 8 Covid vaccines, with the first results for 3 of them (*) released in the last few weeks:
- CanSino, from 6 years (results submitted to China’s drug regulator, but not public);
- * BNT-Pfizer, from 5 years (some results released from age 12);
- * Moderna, from 6 months (some results released from age 12);
- Novavax, from 12 years (with the placebo control group crossing over to vax at 6 months – see my explainer on crossover trials);
- Zy-Cov-D (Zydus Cadila), from 12 years (interim results possible this month);
- Sinopharm’s Beijing vaccine, from 3 years (has results, but not public);
- * Sinovac’s CoronaVac, from 3 years (some results released);
- Bharat’s Covaxin vaccine, had a phase 1/2 trial from 12 years – with approval for phase 2/3 starting age 2 upwards reported; and
- The first phase 1/2 trial soon to start for a new protein subunit vaccine from China’s National Vaccine and Serum Institute will have participants from age 3.
BNT-Pfizer results: There were teenagers aged 16 to 17 in the major trial for this vaccine, and the vaccine has widely been authorized for that age group. However, they were added to the trial later, and there was only data for a small number of them at the time the initial results of that trial were reported. There hasn’t been a further public update on that age group.
Recently, however, Pfizer released some phase 3 results for 2,260 teenagers aged 12 to 15. Signs of immunity were similar to those in people aged 16-25 in the large trial, and none of the vaccinated teenagers got symptomatic Covid-19 (although 18 did in the placebo group). Adverse events were similar to those experienced by people in the 16-25 age group as well. The US FDA authorized the vaccine from age 12, and the EMA (Euro drug regulator) has begun its assessment, too. Pfizer expect that their vaccine will be authorized for 2 years and up by September – and results for pregnant women in the next few months. And their trial of consistency of commercial vaccine lots with the vaccine produced in the major phase 3 trial now goes down to 12 years of age – and that trial has an arm with a lower dose (20μg instead of 30).
Moderna results: In Moderna’s phase 2/3 TeenCOVE trial for 3,235 teenagers aged 12 to 17, they used a broader definition of Covid-19 than in the adult trial. It meant that milder cases of the disease counted, since the disease tends to be mild in young people. In a press release, Moderna said efficacy was 96% from 14 days after the first injection (12 of the participants had Covid-19). They reported there were no safety concerns, but provided no data on adverse events.
CoronaVac results: Sinovac recently released some early phase trial data on their website, in Chinese only. They reported signs of immunity were higher for 3-17 year-olds than for adults, and that a low dose was enough. Adverse events were reported for about a quarter of them, depending on the dose – how many had severe or serious adverse events wasn’t reported. A chart for the neutralizing antibodies in these children and young people was included in their April presentation of results to WHO.
And there were also results of testing the Moderna and Novavax vaccines in infant primates.
Other planned trials:
- A Pfizer trial for children aged 5-11 is planned for 2021;
- The AstraZeneca trial for younger people was paused when the safety review was underway early in April: I don’t know if it that has resumed;
- A Sputnik V trial for 14-18 year-olds was announced;
- J&J announced at the beginning of April that they were expanding their ongoing phase 2a trial to include adolescents aged 12 to 17 – it’s not clear if that’s going ahead;
- Sinopharm registered an immunogenicity and safety trial in China for 3 doses of its Beijing vaccine, for 4,400 people from the age of 3 up. This is a small trial that will look at signs of immune response and safety outcomes, and compare these to the results in the 2-dose trials; and
- The European Medicines Agency (EMA) approved a pediatric trial program for Novavax – so far, only the trial for teenagers is underway.
Study results in people with compromised immune systems
Study results of immune responses to Covid vaccination in people with compromised immune systems are coming in steadily now. There’s a mixture of good and, as expected, not-so-good news, too. The not-so-good news is a major reason that achieving community-wide protection against infection is so critical: vaccination cannot work as well for many of the people who are most vulnerable to the virus. So far, I’ve seen studies in people on dialysis, people with transplants, and people with cancer.
The most studies have been in people on dialysis and with kidney transplants. The team from #NephJC, the Twitter online journal club for nephrology, are maintaining excellent coverage of these, as well as resources generally on Covid vaccination and kidney disease. See their 2 articles:
- An overview of studies and resources curated by Swapnil Hiremath and Joel Topf with a team of contributors and reviewers; and
- An article with charts plotting out the results of studies, by Ed Carr.
The bottom line? So far, the studies are mostly of the BNT-Pfizer vaccine, with some Moderna, too. People with kidney diseases are developing antibodies, but not as well as healthy people, particularly after the first dose. A large study in the US – 1,633 people on dialysis – included some people who had the J&J vaccine as well as people receiving BNT-Pfizer and Moderna: the overall rate of people with weaker signs of immune response was 22%. People who were sicker had weaker results than those who were healthier.
Immune responses are much lower for people with transplants, with vaccinated people also more likely to still get Covid-19. (That’s true for people with heart transplants as well: see here, here, and here – that last link is to a US study of 658 people, half of whom had kidney transplants, the other half had liver, heart, lung, pancreas, or multi-organ transplant. A small, uncontrolled study in people with lung transplants reported a higher response from Moderna than BNT-Pfizer.)
Hiremath and Topf write that more options need to be tested for people with transplants:
- “Would a non-mRNA vaccine (eg the Novavax, or the adenovirus vector platform) work better?
- Add a third dose (since there seems to be some additional conversion after 2nd dose)?
- Hold the antimetabolite and then vaccinate? Trade-off of higher risk of rejection”.
A third dose is already being used in some places, so hopefully there’ll be results on this soon. [Update May 14] A trial of a third dose of Moderna vaccine in people with solid organ transplants has been registered in Canada: some results possible in July.
How much vaccine is enough is raised in some of the studies in people with cancer, too – and particularly the need people with cancer to be a high priority group for the second dose.
- USA: 67 people with various blood cancers (BNT-Pfizer and Moderna vaxes) (preprint);
- Israel: 167 people with chronic lymphocytic leukemia (CLL) (BNT-Pfizer vax);
- Greece: 48 older people with multiple myeloma (BNT-Pfizer vax);
- UK: 15 people with chronic myeloid leukemia (CML) (BNT-Pfizer) (preprint);
- UK: 95 people with solid cancers and 56 with blood cancers (151 in total) (1 or 2 doses of BNT-Pfizer vax);
- France: 122 people with solid cancers (BNT-Pfizer vax);
- France: 110 people with solid cancers (BNT-Pfizer vax);
- Israel: 157 people with cancer receiving immune checkpoint inhibitor treatment offered BNT-Pfizer vax (results for 134 who were vaccinated);
- UK: 21 people with myeloproliferative neoplasms (1st BNT-Pfizer vax) (preprint); and
- Israel: 2 studies of lymphadenopathy detected by scanning, in 719 people with solid and blood cancers, and in 137 people with blood cancers (BNT-Pfizer vax).
Studies in other people with immune-related conditions:
- A report from Italy that Covid vaccination doesn’t trigger flares in psoriasis in people taking apremilast; and
- A US and German study in 83 people on immune-modifying drugs (for psoriasis, psoriatic arthitis, rheumatoid arthritis) found signs of immune response after BNT-Pfizer vax were high in people on biologics, but lower in people on methotrexate – they suggest temporarily modifying (or even stopping) methotrexate temporarily should be studied. (Preprint)
I tweet about these studies and recently began tagging them: you can keep up with those on Twitter via this search – and then click on “Latest”, to see them in reverse chronological order. A proviso here: my searches for these studies are not as comprehensive as my searches for clinical trials and effectiveness studies.
Vaccine combinations
It’s called heterologous prime-boost vaccination: it’s where instead of first (“prime”) and second (“boost”) injection of the same vaccine, they’re 2 different ones. The theory is that this could widen immune responses more than same-same vaccination.
There are now 9 combinations being studied, and they’re listed in chronological order from when they were made public below. There might be results in the next few weeks for the trials alternating the AstraZeneca and Sputnik Adenovirus-26 vaccines, and some early results are out now from the UK’s Com-COV trial. Since my last post, there are 3 new trials of combinations (marked * in the table below). In addition, the Moderna and Novavax vaccines were added to the Com-COV trial.
Com-COV’s first results are for adverse reactions and other preliminary safety data on the 463 people randomized to the 28-day dosing interval for alternating BNT-Pfizer and AstraZeneca (AZ) vaccines. People have to be 50 or older to join, and this group ranged between 50 and 69 years (median 57). That means their adverse reactions would be lower than for the general population, because reactions are so much stronger in younger people for these vaccines.
A couple of things are striking in these results. Firstly, this is the first standardized study of both these vaccines at once – and it confirms that the AZ vaccine’s adverse events are higher than for BNT-Pfizer. Secondly, overall, the combinations had higher rates of adverse events than the same-same course.
Update May 19:
| Vaccines | Schedule | Participants | Location(s) | Research phase |
| Sputnik V: rAd26-S & rAd5-S Sputnik Light: rAd26-S | rAd26-S first; placebo control Sputnik Light: rAd26-S alone | 19,866 Sputnik Light: 110 people | Russia | Phase 3 trial, results. Sputnik Light: in phase 1/2 trial. |
| Oxford-AZ; Sputnik Ad26 | Alternating | 100 people | Belarus, Russia | In phase 1/2 trial. |
| Oxford-AZ; Sputnik Ad26 | Alternating | 100 people | Azerbaijan | In phase 1/2 trial. |
| Oxford-AZ; Sputnik Ad26 | Alternating | 100 people | Not specified (near Azerbaijan) | In phase 1/2 trial. |
| Oxford-AZ; Imperial College saRNA | Single shots; alternating combination; usual 2-shot course | 6 groups; 22 mice each | UK | Preclinical, results. Combos had highest response, followed by usual Imperial College course. |
| Oxford-AZ; BNT-Pfizer; Moderna; Novavax | Alternate boost starting with AZ or Pfizer (8 or 12 weeks apart) | 1,050 people | UK | In phase 2 trial called Com-Cov. First results. |
| ChAdV68-S; ChAdV68-S-TCE; SAM-LNP-S; SAM-LNP-S-TCE (ChAd = chimp adenovirus, SAM = self-amplifying mRNA/saRNA) | ChAds first then SAMs; SAMs first then placebo | 140 people | USA | In phase 1 trial. |
| *CanSino; ZF2001 (protein subunit) | CanSino followed by ZF2001 or a flu vax as a control group | 120 people | China | In phase 4 trial. |
| *Oxford-AZ; BNT-Pfizer | AZ followed by Pfizer | 600 people | Spain | In phase 2 trial called CombiVacS. First results. |
| *Oxford-AZ; BNT-Pfizer; Moderna; (?) Sputnik | Alternate boost starting with AZ | 600 people | Italy | Trial not yet underway. |
The landscape after new data: 11 vaccines with phase 3 results
Before we get to the data on vaccine efficacy, there was a cluster of reports in the last few weeks of how many doses of some Covid vaccines have been distributed (though not necessarily administered) globally – enough to give us a rough idea of what’s mostly being used so far:
- BNT-Pfizer: 450 million (as of May 6)
- AstraZeneca/Covishield: 300 million (end of April)
- Sinovac’s CoronaVac: 200 million (end of March), 260 million (recent WHO report)
- Moderna: 132 million (as of April 12)
- Sinopharm Beijing: 65 million (recent WHO report)
- CanSino (single dose course): Around 40 to 50 million*
- Sputnik V: 33 million (end of March, produced in Russia)
- J&J (single dose course): More than 20 million (end of March)
- Covaxin: 17 million (as of April 18, India only – some also exported)
Sources: Axios, Bridge Consulting, Financial Times, Hindustan Times, J&J, Moderna, Reuters, US News, WHO (* No global total found: based on proportion of China’s vaccine sales (Bridge) and the gap between total Chinese vaccines (Axios) and WHO estimates for Sinovac and Sinopharm.)
Roughly half of the vaccine used globally up to now are probably from China, though the vaccines used in different parts of the world vary a lot. (See Bridge’s assessment of the sale and donation of China’s vaccines outside the country here.)
Critical caveats on the data presentation to follow:
With a single exception, each vaccine has only been tested alone, and we don’t have a rigorous basis for comparing them. On the other hand, with 11 vaccines around the world with phase 3 results, it’s hard to keep track of them and keep them in perspective. I decided an oversimplified “sketch” of this landscape was more likely to help with that perspective, than mislead. But keep in mind:
- The number of people in the trials, and the quality of those trials (and the amount of data available), all vary greatly. That means that there’s a great deal more certainty for some vaccines than others.
- Vaccine efficacy is a relative reduction in the size of risk of disease – not an absolute drop in percentage points of a risk that is 100%.
- The participants can be very different. For the vaccines which have much higher rates of adverse events in people under 55 than over, the age spread in trials could affect that considerably.
- Some of the vaccines have been tested against variants of concern that reduced their efficacy, while others have not.
- Some of the vaccines reach their peak efficacy sooner than others, and their efficacy rates are likely to improve when later results come in.
The data behind some vaccines is very preliminary – sometimes just a few data points in a press release. So vaccines will shift positions, sometimes even a lot: they’re like counters that could move around on a board.
The top left is where vaccines with high efficacy and low rates of adverse events would sit – and the bottom right is lower efficacy and higher adverse events. They all “work” though. The evidence I used to manually position the counters is in the table below. They were numbered in the order in which efficacy results for them were made public.
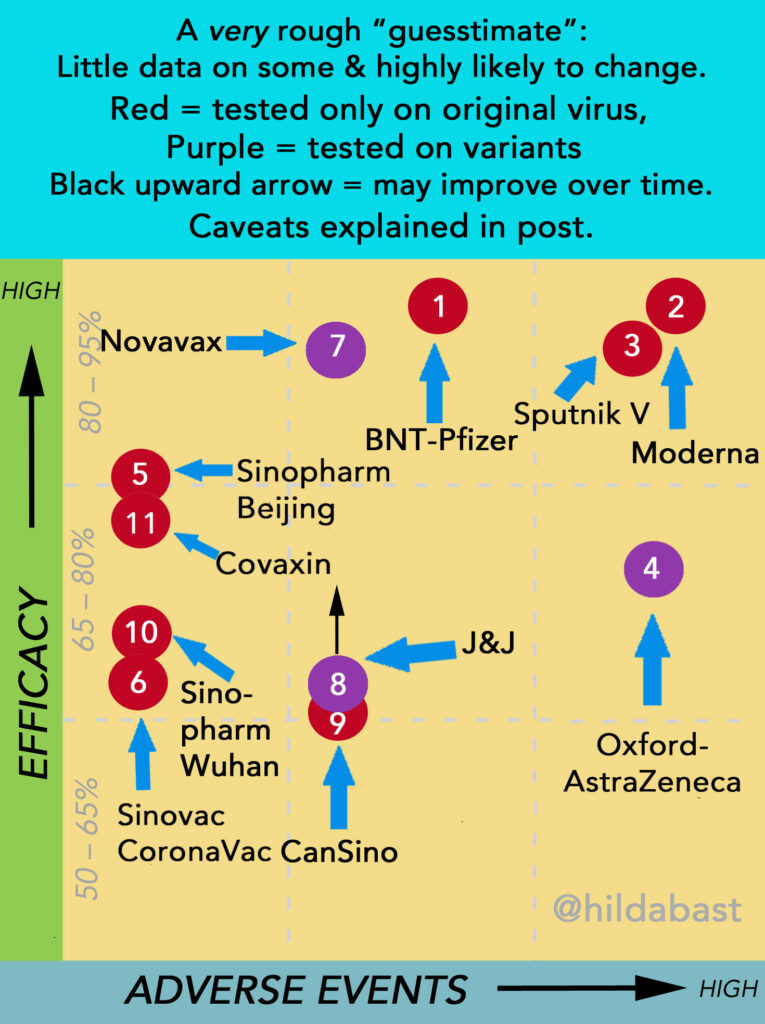
| Key | Vaccine | Efficacy (CI*) | Data |
| 1 | BNT-Pfizer | 95% (90-98) At 6 mths: 91% (89-93) | >43k people (half on placebo) Reviewed & detailed reports public; protocol public Evidence: strong |
| Most common systemic adverse event: fatigue (56%) Most commonly severe: fatigue (4%) | |||
| 2 | Moderna | 94% (89-97) At 6 mths: 90% (?) | >30k people (half on placebo) Reviewed & detailed reports public; protocol public Evidence: strong |
| Most common systemic adverse event: fatigue (65%) Most commonly severe: fatigue (10%) | |||
| 3 | Sputnik V (Gamaleya) | 92% (86-95) | >22k people (3:1 placebo) Reviewed & detailed report public; protocol not public Evidence: moderate-strong (reservations about methodology); adverse events not routinely solicited in a representative group in phase 3, data from San Marino study |
| Most common systemic adverse event: asthenia (fatigue) (32%) Most commonly severe: asthenia (around 5%) | |||
| 4 | Oxford/AstraZeneca | 76% (68-82) (US/LatAm trial) Other trials: 63% (52-72) 76% (59-86) (delayed 2nd dose) (Standard dose, February data, includes “UK”, “SA” strains) | >17k people in grouped trials (half in control group, half on varied vaccine regimens), >32k in US/LatinAmerican trial (two in vaccine to each in placebo) Reviewed & detailed reports public; press release only for US/LatAm trial; protocols public Evidence: strong from US/LatinAmerican trial, though weak to moderate from group of trials with reservations about methodology; adverse event data from MHRA, rare risk from EMA |
| Most common systemic adverse event: fatigue (62%) Most commonly severe: malaise (4%) Thrombosis & Thrombocytopenia Syndrome (TTS): 1 in 100,000 | |||
| 5 | Sinopharm – Beijing | 81% (67-89) (up to age 60) | Up to 30k people (half on placebo) Reviewed by WHO with some details public; protocol not public Adverse event data from earlier phase trial Evidence: potentially strong (too little known) (Other trials in progress) |
| Most common systemic adverse event: fever (4%) None severe | |||
| 6 | CoronaVac (Sinovac) | 50% (35-62) (Anvisa, Brazil) 84% (65-92) (Turkey) | Brazil >12k people (half on placebo) Reviewed by Anvisa & WHO, basic details public; protocol public Turkey >10k people (half on placebo) Reviewed by WHO; protocol not public Evidence: moderate (further trials in progress) |
| Most common systemic adverse event: fatigue (<2%) No severe event reached 1% | |||
| 7 | Novavax | 90% (80-95%) (UK trial only, including “UK” strain) 96% (Original strain only) (74-99.5) | >14k people (half on placebo) (UK trial) Press release only; protocol public Evidence: moderate; adverse event data available for small early phase trial and conference presentation of UK trial (large trial in progress) Note: the results from a small phase 2b trial in SA showed it worked, but with much lower efficacy because of the “SA” strain (perhaps 49% or 60%). However the evidence was very weak. |
| Most common systemic adverse events: fatigue/headache/muscle ache (>30%) Most commonly severe: fatigue (around 3%) | |||
| 8 | J&J | 66% at 28 days (Including “SA” strain) (55-75) ~70% after 56 days; 72% at 28 days USA (58-82) | >43k people (half on placebo) Reviewed, detailed report; protocol public Evidence: strong (further large trial on 2 shots in progress); rare risk from CDC & EMA |
| Most common systemic adverse event: fatigue (38%) Most commonly severe: fatigue (1%) Thrombosis & Thrombocytopenia Syndrome (TTS): about 1 in 400,000 | |||
| 9 | CanSino | 69% at 14 days (?) 65% at 28 days (?) | ca 40k people (half on placebo) Press release only; protocol not public Adverse event data only available for small early phase trial Evidence: potentially strong (too little known) |
| Most common systemic adverse event: fatigue (34%) None severe reached 1% | |||
| 10 | Sinopharm – Wuhan | 73% (?) 2nd trial stopped for lack of efficacy | Up to 30k people (half on placebo) Reviewed, negligible details public; protocol not public 2nd trial 6k people (half on placebo) No data released Adverse event data from earlier phase trial Evidence: potentially strong (too little known) |
| Most common systemic adverse event: fever (5%) None severe | |||
| 11 | Covaxin | 78% (61-88) | Under 26k people (half on placebo) Press release of second set of interim results only; protocol not public Adverse event data from earlier phase trial Evidence: potentially strong (too little known) |
| Most common systemic adverse event: fatigue (3%) None severe |
What else is new for the 11 vaccines
Janssen-Johnson & Johnson (J&J)
The J&J vaccine had a major setback at the end of March when a US factory had to discard close to 15 million doses because of manufacturing problems, leading to a major ongoing supply. In April, the EMA listed Thrombosis and Thrombocytopenia Syndrome (TTS) as a very rare side effect of this vaccine, and a few days later the CDC decided to do the same in the US.
With 28 reported cases and over 11 million doses administered globally, the rate is about 1 in 400,000. Kai Kupferschmidt and Gretchen Vogel report in their recent report, and Roxanne Khamsi has written an overview of the theories about what’s causing TTS – which has so far only been reported in this and the AstraZeneca vaccine (both of which are adenovirus-based).
In other news:
- The EMA released its assessment report for the vaccine, and J&J’s article on their phase 3 trial was published in the New England Journal of Medicine.
- The South African Medical Research Council reported that over 366,000 healthcare workers had been vaccinated by May 6 in their J&J vax implementation study, Sisonke, with more than 80% protection against severe Covid-19 and death.
- Although there are continuing supply problems and the US has, as predicted, vaccinated the most enthusiastic population, the pause on the J&J vaccine does not appear to have increased vaccine hesitancy.
This vaccine is listed by WHO and distributed in the COVAX scheme.
Records in my collection for this vaccine here. Includes one laboratory on the D614G variant (none on variants of concern), as well as clinical trial data on B.1.351 (“SA” variant of concern) in the international phase 3 trial.
This vaccine also in the sections on community impact sections, immunosuppression, and trials in children.
CanSino
CanSino is a single-shot viral vector vaccine, based on Ad5 (trade name, Convidecia). The manufacturers are concerned, though, that immunity may wane, and are considering a 6-month booster. They report that they have not had a report of TTS after their vaccine.
In other news, Pakistan’s National Institute of Health will be preparing the vaccine locally, based on raw materials provided by CanSino. The first vaccines are planned to be roll off the line in May. And the vaccine got the green light from Chinese authorities to start a trial of an intranasal version of the vaccine.
This vaccine is being evaluated for WHO listing. Records in my collection for this vaccine are here – no studies on variants. A summary of the minimal phase 3 data released so far for this vaccine is in my March post.
This vaccine also in: the trials in children and vaccine combination trials sections.
BNT-Pfizer
As of May 6, more than 450 million doses of this vaccine had been supplied globally, and their new goal for 2021 is 1.8 billion. That is the largest supply of a single Covid vaccine globally, overwhelmingly in North America and Europe.
New recent trial results for this vaccine include preliminary results in 12-15 year-olds (see section on children above), and 6-month follow-up of the phase 3 trial. Some longer term outcomes are only available in a press release, including:
- Efficacy against symptomatic Covid-19: 91.3% (CI 89-93), based on 77 people in the vaccine group and 850 in the placebo arm;
- Efficacy against severe disease (FDA definition): 95.3% (CI 71-99.9), based on 1 vaccinated person and 21 in the placebo group;
- Among the 800 trial participants in South Africa, 9 people – all in the placebo group – developed Covid-19, and 6 of them were infected by B.1.351 (“SA”) variant of concern.
In other news for this vaccine:
- A trial for 550 participants is underway testing a lyophilized (freeze-dried) version of the vaccine is at least as good as the original version (based on signs of immune response and safety).
- The US FDA authorized the vaccine’s use from 12 years of age, and Pfizer has applied for full marketing approval of the vaccine.
- EMA is listing possible facial swelling in people who have had dermal fillers injected as a potential side effect of this vaccine.
- No regulator has flagged of perimyocarditis (also called myopericarditis), an inflammation around the heart, as a safety concern for the vaccine. EMA has said they are investigating, but haven’t seen a link. However, a study has been registered in Israel to investigate the prevalence. Most people recover from this condition spontaneously, though some need treatment. It has been flagged as a very rare vaccine-induced adverse event after smallpox vaccination.
- BNT is setting up regional headquarters and manufacturing facilities in Singapore, and they announced that their partner in China, Fosun, aims to produce a billion doses of the vaccine.
This vaccine is listed by WHO and distributed in the COVAX scheme. Records in my collection for this vaccine here. Includes 33 laboratory studies on variants (including all variants of concern), and trial or community impact data for B.1.1.7 (“UK”) and B.1.351 (“SA”) variants of concern.
This vaccine also in: the trials in children, people with immunosuppression, and vaccine combination trials sections.
Oxford-AstraZeneca
These have been difficult weeks for this vaccine, as concerns about Thrombosis and Thrombocytopenia Syndrome (TTS) saw several countries set it aside completely, and supply problems continued. While they had originally planned on supplying 3 billion doses globally in 2021, by April 30 the quantity from all suppliers combined was 300 million doses (considerably less than BNT-Pfizer).
In April, the EMA listed TTS as a very rare side effect, and their current estimate of the risk is about 1 in 100,000 – 1 in 50,000 for those under 50, and 1 in 200,000 for those over 70. The UK has reported a fairly similar rate, though as Kai Kupferschmidt and Gretchen Vogel report in their recent overview, reporting rates in individual countries varies – in part, because it’s rare and so fluctuations are expected, and in part, a reflection of differing likelihood internationally that this complex phenomenon will be diagnosed and reported. As Roxanne Khamsi writes, there are still several theories about the cause.
But there was great news, too, for the Covid vaccine that’s the second most widely used one globally. The results of the long-awaited large and well-designed US/Latin American trial showed that the earlier smaller problematic trials had underestimated the vaccine’s efficacy (press release). Vaccine efficacy rates in 32,449 people with doses 4 weeks apart were:
- Against symptomatic Covid-19: 76% (CI 68-82);
- In people aged 65 and over: 85% (CI 58-95); and
- No severe or critical Covid-19, or hospitalizations in the vaccinated group versus 8 in the placebo group.
That good news was overshadowed, though, by the drama around the data release. AstraZeneca originally issued a press release with earlier data on March 22, and the NIH mirrored those findings in a release of its own. However, the data monitoring committee contacted the NIH and others expressing concern that misleading data had been released, so the NIH issued a statement about the problem. Anthony Fauci referred to this as “an unforced error” by AstraZeneca. (We never did get to see the data referred to by the data monitoring board, as the final press release was a later analysis.)
The phase 2 trial of the vaccine in South Africa that had such disappointing results for the local variant of concern has reportedly been unblinded. People in the placebo group were offered vaccination with the AZ vaccine, while people who got it in the trial were offered a third dose.
In other news:
- AstraZeneca’s application for FDA authorization has been delayed by FDA requests for additional data.
- Several studies in the UK now confirm the vaccine has had a big impact on risk of hospitalization and death from Covid-19 (see impact section above).
- The Oxford group posted a preprint of results for 54 people living with HIV who had the vaccine – they reported similar signs of immune response as people who are HIV-negative.
- Both the Com-COV trial and a monitoring study in the UK confirm that the AZ vaccine has more adverse reactions than the BNT-Pfizer vaccine.
- The EMA announced it is investigating cases of Guillain-Barré Syndrome that occurred after the vaccine, and Brazil’s drug regulator, Anvisa, suspended its use in pregnant women as a precaution while they investigate a serious adverse event (a pregnant woman’s fatal stroke).
- Technology transfer from AZ to Brazil has been completed successfully, with Anvisa approving the start of local production of the active pharmaceutical ingredient.
- The EU is not taking up its option for further doses of this vaccine.
- Vaccitech, the Oxford researchers’ spin-off company that owns this vaccine, floated the company on the New York Stock Exchange.
The Oxford institute behind this vaccine’s development was also in the news in the last month, because they developed the malaria vaccine that reported high efficacy in a trial (using Novavax’s proprietary adjuvant).
This vaccine is listed by WHO and distributed in the COVAX scheme. Records in my collection for this vaccine here. Includes 5 laboratory studies on variants, including B.1.1.7 (“UK”), B.1.351 (“SA”), P1 (“Brazil”), and B.1.617.1 variants of concern, as well as clinical trial data on B.1.1.7 and B.1.351.
This vaccine also in: community impact data, vaccine combinations, trials in children.
Moderna

The Moderna Covid vaccines aren’t really called M1 and M2 etc! But I’ve mapped out my personal nicknames for them, in case it helps other people keep those vaccines and their official names straight, too. There’s been a flood of new data for these various vaccines.
M1 – the original:
First we got results for 6-month follow-up of 33 people who had been vaccinated back in Moderna’s phase 1 trial – “activity remained high”. Then came a press release announcing the 6-month follow-up of the big phase 3 trial. There were now 900 events – symptomatic Covid-19 – and over 100 people had severe Covid-19. They didn’t give detailed data about this, just that efficacy was “greater than 90%” for symptomatic Covid-19, and “greater than 95%” for severe Covid-19.
By April 13, almost everyone in the placebo group had been vaccinated: so 6 months is the end of randomized efficacy data for this vaccine.
M1a and M1b – adapting for the B.1.351 variant:
First we got encouraging preclinical results of the version against variants. Then a press release of phase 2 results, followed shortly by a preprint: while a booster shot of the original M1 boosted immune response, M1a (mRNA-1273.351) induced a stronger response against the B.1.351 (“SA”) variant of concern.
M2 – next generation and the end of the super-freezing:
This vaccine, officially called mRNA-1283, is meant to be stable in normal refrigeration. It’s being tested against M1 in a phase 1 trial with 100 people, at 3 different doses.
And in other news:
- This vaccine has just been listed by WHO and can be distributed through the COVAX scheme.
- A trial for 37,500 university students in the US started, to study transmission dynamics. The young people who join will get Moderna vax either straight away, or 4 months later. There’ll be testing, and then contact tracing for anyone who gets infected.
- It’s also the vaccine being used to test transmission dynamics in an isolated/self-contained Hutterite community in Canada.
- The vaccine was added to the UK COM-COV trial of alternating doses at different schedules (see vaccine combinations).
- And from CDC monitoring, confirmation from what has been clear from the trials: the Moderna vax is the one with the most adverse reactions.
This vaccine is listed by WHO. Records in my collection for this vaccine here. Includes 10 laboratory studies on variants, including the animal study of the adapted vaccine mentioned above, and B.1.1.7 (“UK”), B.1.351 (“SA”), P.1 (“Brazil”), and B.1.526 (“New York”).
This vaccine also in: community impact studies, trials in children, people with compromised immune systems, and vaccine combinations.
Novavax
The Novavax vaccine has better trial data to support it than the AZ vaccine had when it was authorized in Europe and elsewhere. But getting production up to speed enough for evaluation might be holding it back there. Authorization isn’t an option in the US until the big US/Mexico trial has results. With the slowdown of the coronavirus wave in the US, Novavax isn’t expecting results from their big trial until the end of May – and with production delays because of shortages of materials, they don’t expect to be in full production till late in 2021.
Natasha Loder reports that Novavax expects authorizations to run in this order: UK first, and then South Korea – both of which have factories producing the vaccine. After that, the Europe (EMA), WHO, and then the US. WHO is key to being able to provide vaccines to COVAX. They have signed a commitment to start shipping vaccines for COVAX between July and September, with 350 million doses to come from them. They are still hoping for 750 million doses this year for COVAX from the Serum Institute of India, but that seems over-optimistic.
In other news for this vaccine:
- Data in a conference presentation showed that the rate of adverse events in their UK trial was similar to their early trial – confirming that so far, this vaccine has considerably less adverse reactions than the other very high-efficacy Covid vaccines.
- The protocol for the US/Mexico trial that updates for the crossover trial was released (see my explainer).
- They released preclinical results of a combined Covid-19 and influenza vaccine, that also held up to Covid variants in the lab (preprint).
- The US/Mexico trial was extended to include 3,000 teens aged 12 to 17 – while efficacy results will be slow, data on safety and signs of immunity could be available later this year.
- They began dosing with Novavax in the UK COM-COV trial of alternating doses at different schedules.
Novavax was also in the news in the last month because their proprietary adjuvant used in the Covid vaccine, Matrix M, is also the adjuvant in the malaria vaccine that reported high efficacy in a trial.
With AZ pushed back, if Novavax US/LatAm trial results come soon, as expected, then it will be the next Covid vaccine up at the US FDA.
This vaccine is being evaluated by WHO for inclusion in the COVAX scheme.
Records in my collection for this vaccine here. Includes 2 laboratory studies on variants, including B.1.1.7 (“UK”), B.1.351 (“SA”), and P.1 (“Brazil”) variants of concern, and clinical trial data for B.1.1.7 and B.1.351.
This vaccine also in the sections on trials in children and vaccine combinations.
Sputnik V
- It became clear this month that the developers of this vaccine have not resolved the shortcomings of their early and clinical phases of testing with further work – and that’s a major roadblock for several major drug regulators.
- Quality control of manufacturing emerged as a further serious problem.
- Critical assessments of deficiencies preventing approvals by drug regulators in Slovakia and Brazil escalated into major public conflicts.
- Given the weaknesses of the pre-clinical and clinical trial data publicly available for this vaccine, robust studies of its effectiveness in community use are critical, but none has been reported.
- It’s not yet clear if this vaccine is affected by the Thrombosis with Thrombocytopenia Syndrome (TTS) occurring after other adenovirus-based Covid vaccines.
- There is a high level of disinformation in the vaccine developers’ public campaigning about its vaccine, other vaccines, and drug regulators.
Given the dramatic events of the last few weeks and the blizzard of claims and counter-claims kicked up around them, I needed to comb through it all systematically to gain a perspective on what was happening. I’ve laid the results of that process out in a detailed timeline below, so that you can see why and how I came to the conclusions in the above summary points. Each point is tagged for which of 3 streams it relates to – Slovakia, EMA (EU), or Brazil.
I often stress that we need to keep in mind that the quality of 3 things can be quite separate: the vaccine itself, the clinical trial program for that vaccine, and the behavior of developers. This is particularly critical to keep in mind with this vaccine.
“Developer” throughout could refer to the Gamaleya Institute that created the vaccine, the Russian sovereign wealth fund (RDIF) that organizes its production and deals, or their vaccine-specific communication channels (website and Twitter account).
- April 6 – Slovakia: A newspaper reported that the national drug regulator, the State Institute of Drug Control (ŠÚKL), could not verify the safety or efficacy of the vaccine because there was too much missing data in the dossier submitted, and the doses sent to Slovakia came in a variety of formulations that weren’t all consistent with the version reported in the phase 3 trial – including liquid and lyophilized (freeze-dried) forms, single and multi-dose vials, and differing storage conditions, composition, and method of manufacture. The ŠÚKL had reportedly run tests at an accredited laboratory at the Slovak Academy of Sciences. The Russian developers asked the Slovak government to get the vaccine tested at an EU-certified laboratory and return the shipment “due to multiple contract violations” so they could deliver it to another country. (It’s not clear if another country has since received these doses.)
- The same day – European Medicines Agency (EMA): The EMA’s planned site inspections of production facilities in Russia were delayed by Russia. The EMA reportedly will also need to establish that Sputnik V trials followed good scientific practice, as there have been some (apparently unverified) reports that raised ethical concerns. (EMA and WHO are planning inspections from May 10 to the first week of June.)
- April 9 – Slovakia: Hungary’s Foreign Minister confirmed that Hungarian laboratories would be undertaking additional tests of Sputnik V doses for ŠÚKL.
- Some time in the first week of April – Brazil: The President of Brazil reportedly spoke with the President of Russia to request his help in resolving the problems Brazil’s regulator, Anvisa, was having in getting access to essential data about the vaccine.
- April 16 – Brazil: Russia delayed the site inspections Anvisa, Brazil’s drug regulator, had booked for production and quality control facilities in Russia, without giving a reason. A leaked report suggested that Anvisa had grave concerns about the lack of data provided for the vaccine, including basic biological and safety data.
- April 21 – Brazil: The Anvisa inspectors visiting Russia were denied permission to inspect the quality control procedures at Gamaleya.
- April 22-23 – Brazil: Anvisa inspectors completed their inspection of the 2 factories in Russia that were to put the vaccine together and bottle doses for Brazil, but as they were not allowed to visit other factories, they could not sign off on components or doses produced elsewhere. For those 2 factories, validation processes for filtration/sterilization had not been completed; for 1, the manufacturers still need to ensure sterility of production. They could not verify adequate manufacturing conditions for either of the 2 factories.
- April 27 – Brazil: Anvisa, Brazil’s drug regulator, announced in a live-streamed meeting that they could not approve importation of the vaccine, because they could not confirm the vaccine’s 2 components were safe and effective as there were serious deficiencies and causes for concern in the data submitted to it, including the lack of key certification of manufacturing standards and quality control. (The video of the meeting is here: the major technical presentation is here, and the report on manufacturing facilities is here.) They were left with serious questions about:
- vaccine development;
- biological action of the vaccine;
- biases in the efficacy data and lacking adverse event data in the phase 3 clinical trial;
- consistency in potency and quality/purity of the vaccine – this included a concern about replication-competent adenovirus (RCA) detected in batches of the Adenovirus 5 (Ad5) dose (the second injection);
- compliance with expected manufacturing practices – and they could not identify where the raw biological components were manufactured (with no certification of manufacturing practices used in their production).
- Sputnik same-day response – Brazil: The developers issued a statement in Portuguese (with an English translation the following day). They claimed Anvisa’s actions were political and baseless, and alleged further:
- That Anvisa’s decisions contradicted a safety report by a Brazilian agency lower in the regulatory chain (note: that agency also has a limited remit);
- Anvisa had been informed not only that there was no RCA, but that none was possible;
- Argued that they had a purification system that was superior to those used for other vaccines; and
- Made several claims about the effectiveness of their vaccine relative to other vaccines (which we’ll come to later).
- The same day – on Twitter: The Sputnik V account tweeted a claim a study by them showed “there are significantly more deaths following vaccination with BNT-Pfizer than with AstraZeneca vaccine per 1mn administered doses”. Carl Bergstrom rebutted the claim here, concluding “It’s awful propaganda from Sputnik”. That’s because, for example, it quotes overall deaths after vaccination (not vaccine-related deaths), and the BNT-Pfizer vaccine was used far more often, and preferentially in elderly people (who are more likely to die on any given day, vaccinated or not). (Note: American use of mRNA vaccines swamps vaccine use globally, and BNT-Pfizer came first – that makes the BNT-Pfizer vaccine more of everything.)
- The same day – EU: The EU released a report on Covid-19 disinformation, pointing to multi-pronged Russian campaigning about the Sputnik vaccine (including Twitter), spreading conspiracy theories and disinformation, and seeking to undermine trust in the EMA.
- The Sputnik vaccine Twitter account responded that the Sputnik vaccine was the victim, not the instigator, of disinformation and attacks.
- April 29 – Brazil: The Sputnik Vaccine Twitter account turned to Anvisa again, tweeting that it had made “incorrect and misleading statements without having tested the actual Sputnik V vaccine”, disregarded a Gamaleya assurance that there was no RCA, and that “their lawyers would be in touch”, and claimed that Anvisa “admits mistake”, because their spokesperson had made it clear to reporters that their assessment had been based on a dossier provided by the developers. That’s not admitting a mistake, it’s just explaining a very normal process – drug developers are typically expected to shoulder the responsibility and associated costs of demonstrating standards have been met, and assessment by developer-provided dossiers is common.
- April 30 – Brazil: Anvisa held a live-streamed press conference to address the Sputnik group’s allegations, at which they showed excerpts of documents from the vaccine’s dossier and broadcast relevant segments of videoconferences between Anvisa and the developers. (The recording is on YouTube.) They showed:
- Anvisa expects an adenovirus vaccine to have the maximum amount of RCA set by US FDA, but the Sputnik developers set the bar far lower. (Virologist Angie Rasmussen tweeted that the Russian level “is coincidentally the limit of detection of many plaque assays in terms of reliably determining titer”, suggesting that their laboratories might not have had the technology needed to provide data to the FDA standard.)
- They showed an excerpt of the Sputnik dossier with results for 3 batches of the Ad5 vaccine, registering “less than” the unacceptably higher level of RCA.
- Anvisa then had a videoconference with them (it was on March 23), where the developers explained why they didn’t test the Adenovirus 26 (Ad26) vaccine for RCA at all, essentially stating it was impossible for there to be RCA, if I understand their argument correctly. (Side note: I checked the EMA report on the J&J vaccine, which is also Ad26-based. J&J make somewhat similar statements – but test for RCA anyway, as it is expected.)
- Anvisa played another part of the videoconference, where someone from Anvisa asked why didn’t the developers use different materials when they realized there was the potential for RCA with the Ad5 vaccine: the answer was they could have gone back to the drawing board to resolve this, but they didn’t have enough time.
- The next part they played showed the Russian scientists appreciated they were being asked to go back and test: the Sputnik team requested a letter outlining every extra issue they needed to document. Anvisa sent the letter: they got a reply on March 26 that, they said, did not answer their questions.
- Same day – Brazil: Sputnik tweeted:
- “In its press conference today, Anvisa confirmed that it did not find any replicated adenovirus (RCA) in Sputnik V but was concerned about the Russian theoretical regulatory limit for that parameter”, but not acknowledging that they had reporting a non-zero amount themselves – and of course, the testing level reported was not “theoretical”.
- They also claimed that no RCA had been found in any batch (which contradicts the report in their dossier showed by Anvisa), and that their letter of March 26 confirmed this – accompanied by a pull-quote that doesn’t actually say that exactly. So I guess now we can expect Anvisa to release the actual letter?
- Same day – Slovakia: Slovakia’s Ministry of Health published the contract for purchasing Sputnik V, which ŠÚKL pointed out required extensive and detailed testing and documentation for the vaccine (see Schedule 6) and did not require the regulator to use a specific type of laboratory for their own tests.
- (One of Sputnik V’s allegations on April 9 had been that “Unfortunately, in violation of existing contract and in an act of sabotage the State Institute of Drug Control ensured that Sputnik V tested in a laboratory which is not part of the EU’s Official Medicines Control Laboratory network even though OMCL laboratories were available”.)
- (By the way, the price in the contract was $9.95 dose, confirming that the price per dose of Sputnik V can be higher than that for the BNT-Pfizer vax.)
There was a lot of media focus on one of the issues here: RCA being detected in the vaccine. What is the potential health significance of that? Virologist Angela Rasmussen told STAT News that “in this case, it’s not being delivered via the respiratory tract, so you wouldn’t necessarily expect these viruses to cause a respiratory disease. These are being delivered intramuscularly, which is not the way that adenoviruses are normally transmitted. That means there could be potentially unpredictable results of what could happen, particularly in recipients who might be immune compromised or have some type of immune dysfunction”. She tweeted that the FDA standard ensures there is virtually no RCA, because viral replication is exponential, “So 10 viruses can become 100, 1000, 10000, 100000…you get the idea”.
Whether or not there is RCA is vaccines people are using, and whether or not that caused anyone harm, it is part of a picture reported by Anvisa and ŠÚKL of patchy information and some inadequate standards in quality control in manufacturing this vaccine. The EMA is also apparently dealing with a dossier with too much missing information.
Even leaving aside the RCA and manufacturing issues, the other issues alone would be enough to result in a regulatory red light for a vaccine – including denying a regulator permission to inspect facilities it deems necessary.
What about the other issues? There’s very little data available about this Covid vaccine’s development and clinical testing program in public. For example, no preclinical study has been published at all (and no regulator, as far as I know, has released early biological and developmental data either). Those studies aren’t just important for testing before first-in-human studies. They’re also critical for assessing biological and safety data about toxicity.
With Covid vaccines, for example, animal tests are a critical part of building the case that the vax doesn’t cause antibody-dependent enhanced disease (the theoretical risk that a breakthrough case of Covid-19 could be more severe than if the person wasn’t vaccinated). Regulators like the FDA, EMA, and the others discussed here require a minimum amount of information like that before they will green light a first-in-human trial. From what these regulators are saying, it’s not just that this data hasn’t been published: it seems that they don’t have access to it either.
That kind of data also forms an important part of the assessment before authorizing general rollout. See for example the EMA’s assessment report for the first Covid vaccine it published in December – and what you see in there is just a summary of the massive amount of data assessed. The early phase clinical trials are critical as well.
For Sputnik V, the early phase clinical trials were published, but they weren’t reassuring. Here’s some of my criticisms when they came out:
There was no placebo group, which isn’t that unusual for phase 1/2 trials. What is odd, though, is that they didn’t randomly allocate people to the groups. The 38 people getting Gam-COVID-Vac regimes were mostly men – only 6 were women, and they were in the group getting 1 of each, leaving 2 of the groups 100% male. And they were a very young group of healthy people – for the 1 of each vaccine vector group, the average age was 26.4. They clocked up the highest rate of fever of any Covid-19 vaccine trial so far – everyone: 95% had mild fever, and the 1 other person had a moderate fever. Around half had headaches. There were no severe or serious adverse events.
There were 2 trials: the one above with 38 people getting Gam-COVID-Vac, the Sputnik V that went to phase 3 trial. It needs to be frozen (although it doesn’t need the super-freezers that the first generation of BNT-Pfizer and Moderna vaxes do). In another 38 people, they tested Gam-COVID-Vac-Lyo. That was a lyophilized (freeze-dried) version, developed because it would be easier to distribute and use in many parts of Russia and the rest of the world. They concluded from these small studies – which broke the groups down into people getting 2 injections of either the Ad26 or Ad5 components, or 1 shot of each – that the lyophilized version didn’t work as well.
So the 2-component regime of the frozen and “Lyo” versions were tested on just 20 people each before the phase 3 trial, which only tested the frozen version only. I haven’t seen any other studies comparing frozen and “Lyo” versions. To check if the report from Slovakia that some of the doses delivered to them were lyophilized, I Googled that, and found a press release from the developers mentioning the lyophilized version was “launched” for international use in November 2020, and it’s being used in Pakistan and reportedly delivered to India as well.
Which brings us to the concerns about the phase 3 trial. Anvisa expressed concern about gaps in safety and adverse events data. That’s one of the issues I criticized too when the trial report was published:
There is a problem with the way adverse reactions were monitored, though. They relied on spontaneous reporting, not formally soliciting particular adverse events routinely for a representative group (see my explainer on this). It means that we can expect adverse reactions are likely to be underestimated. (They weren’t reported in this paper.)
Anvisa was also concerned about biases in the efficacy data, including that their method of identifying the key efficacy outcome – symptomatic Covid-19 – may not have been reliable enough. That adds up to a lot of issues the developers have to address, and it certainly doesn’t support the Sputnik claim that Anvisa’s decision is “of a political nature and has nothing to do with the regulator’s access to information or science”.
What about their various claims about Sputnik V being proven to be the best vaccine in several countries? In the press release, that centered on 3 sets of data, one each from Hungary, Mexico, and Argentina. And there’s another claim they are circulating about “real world evidence” from Russia. Note that in my assessment of “real world studies” (above), I’ve identified no rigorous studies of Sputnik V effectiveness. A rigorous study needs to try to take account of factors other than the vaccine that could affect the risk of infection – like the rise and fall of surges, lockdowns, and the vaccine’s availability in geographic areas across time – and the risk of poor outcomes – like the age and health of the people getting access to vaccination.
There has been a useful study of adverse events from San Marino, though: as I discussed, it places this vaccine at the high end of the Covid vaccine spectrum for adverse events. And I agree with Kai Kupferschmidt and Gretchen Vogel’s conclusion about this vaccine and TTS: that while the developers have said there have been no reports of clotting disorders after the vax, “it is unclear how many people have received the vaccine, and many of the countries where it has been distributed may be hard-pressed to diagnose the syndrome”.
Let’s look at the 4 claims being made for Sputnik, and the data behind them.
Claim 1: Hungary – “the best safety…and efficacy”
The source is a table in a Facebook post by the Hungarian government. Here is the Sputnik Twitter post (which is the same as its press release) side by side with the Hungarian Facebook post it claims to represent.

The first Hungarian column of numbers is missing from Sputnik’s translated re-configuration. It’s the number of doses, and leaving it out means it’s not immediately obvious, for example, that the BNT-Pfizer accounts for over half of vaccine doses administered in the country (53%), while Sputnik V accounts for less than 4%. As with the claim we discussed above, this matters, because it means the comparison they’re making isn’t fair.
Hungary began vaccinating in December 2020, with BNT-Pfizer vaccine only, and elderly people and those with chronic illness in its vanguard. Chronic illness will disproportionately scoop in people who are more likely to die of both Covid-19 and other causes, and people with suppressed immune systems for whom vaccination, predictably, will often be less effective. The country began using Sputnik V in February, and it was designated for people under 75 without chronic illness. Older and sicker people are, of course, more likely to get sick or die on any given day, vaccinated or not. There’s also no accounting for the factors other than vaccines that could influence the risk of infection and poor outcome. The data they’re presenting cannot prove what Sputnik V’s communication claims it does.
Claim 2: Mexico – “best safety profile”
Here’s the same claim on Twitter as the press release. The claim is that it’s the safest vaccine, based this source of adverse events data and this one for vaccine deliveries (from COFEPRIS, Mexico’s drug regulator and the Mexican government). However, the rates per thousand doses aren’t calculated by COFEPRIS: the footnote indicates they were by Sputnik, based on “assuming that vaccines that arrived first were applied first” – which isn’t a reasonable assumption: doses can be sitting in freezers and fridges well before they’re used.
The BNT-Pfizer vaccine was authorized in December; Sputnik V, in February – the first people getting the Russian vaccine at the end of February. The Sputnik claim puts its vaccine and the ones from AZ and Sinovac at a fairly similar level for adverse events potentially related to vaccination – well under 1 per thousand doses. BNT-Pfizer, though, comes in at 3.6 per thousand. And again, there are serious problems with this use of data.
There were only 2 deliveries of Sputnik vaccine: 200,000 doses on February 22 and 200,000 on March 10. That second batch is too close to March 20 to have been both used, and have all post-vaccination event reports in and analyzed. So the Sputnik calculation is diluted with that second half of doses.
There were close to 3.9 million doses of BNT-Pfizer vaccine, between December 23 and March 16, and they arrived in many batches across that time. To get the number as high 3.6 per thousand, Sputnik seems to have counted all the adverse events reported up to March 20, but not all the doses delivered. (The number is around 3.0 per thousand if you count them all.)
It’s impossible to know what a fair comparison of numbers here would be based on those sources – and, of course, that would have to deal with the inevitable skew of older higher risk people for the BNT-Pfizer, because Mexico, too, began vaccinating with the highest risk people first – for nearly 2 months with only BNT-Pfizer before the Russian, or any other, vaccine arrived. Again, there’s no accounting for the factors other than vaccines that could influence the risk of infection and poor outcome. The data they’re presenting cannot prove what Sputnik V’s communication claims it does.
Claim 3: Argentina – “the most efficient” (of 3 vaccines)
This is the third of the claims in the press release. There’s no source given for this one, but here on Twitter they’re quoting a Twitter thread as the source – which in turn only identifies the author, not the source. It’s data derived from the national monitoring system, showing that the rate of Covid-19 from 14 days after the first shot for Sputnik is 0.27%, while it’s 0.46% for AZ (Covishield) and 0.49% for Sinopharm.
This is raw data that doesn’t take account of potential differences between the people getting the vaccines: all or some of the differences could be because of the vaccines – but other factors could contribute, too. However, it’s less problematic than the 2 we’ve just looked at. It’s Covid-19 rates from 14 days after a single dose, although it doesn’t say how second doses are dealt with in this data.
There’s also no accounting for the factors other than vaccines that could influence the risk of infection and poor outcome. The data they’re presenting cannot prove what Sputnik V’s communication claims it does.
Claim 4: Russia – “97.6% efficacy…the most efficient Covid-19 vaccine in the world”
This comes from a press release, with an analysis, apparently by the developers, based on routine data collection from the Ministry of Health. The press release says that the study will be published in a peer-reviewed journal in May. It’s data from December 5 to March 31, based on linkage between a register of 3.8 million vaccinated with Sputnik, and a register of people diagnosed with Covid-19. The rate of infection for the vaccinated people counted diagnoses from the 35th day after the first Sputnik vaccine. And the rate for unvaccinated people is calculated based on infections and the size of the unvaccinated population.
While 3.8 million is a lot of people, it’s a very small proportion of the Russian population (146.7 million) – under 3%. The risk is very high that this is an atypical group of, and we don’t know if they are atypical in ways that affect their risk of getting, or being diagnosed with, Covid-19. Again, there’s no accounting for the factors other than vaccines that could influence the risk of infection. So this data cannot prove what Sputnik V’s communication claims it does either.
What now? Obviously, what happens with EMA and WHO in the coming weeks should be revealing. There are several routes for addressing the issues that have been raised in the last few weeks. Manufacturing processes can be improved and the necessary certifications gained. Other countries are developing manufacturing capacity for this vaccine as well.
Additional laboratory studies could be done, including animal studies. And more clinical studies gathering safety data and evaluating signs of immunity could be done, too – as well as robust effectiveness studies. Argentina is a likely source of studies. The data Sputnik cited for one of the claims above reported more than 2.6 million doses had been injected there a few weeks ago – and there’s been at least 1 more large shipment of Sputnik dose 1 since then. We’ve already seen results of a quite large immunogenicity study in healthcare workers from CONICET, a major public research agency in Argentina (preprint).
It’s a terrible situation for drug regulators, with enormous political and social pressure to authorize this vaccine. Studies from other countries are going to be pivotal for this vaccine. The vaccine’s developers have shredded their credibility for many people in the last few weeks. While just the fact that the Sputnik studies have been published in The Lancet is enough to give a lot of people confidence, those papers reveal a lot of reasons to question the Russian clinical trial program. I expressed concern, too, that the protocol for the phase 3 trial still isn’t public.
In an op-ed in BMJ, Chris van Tulleken takes us through the troubled history of those Lancet papers: concerned researchers still have not been able to get access to the data the authors and journal said would substantiate figures in the paper for the early trials – they’ve been trying since early September. And he points to what he says appear to be data anomalies in the phase 3 trial paper as well. (Both the Lancet papers have had errata notices for data errors.) van Tulleken poses some serious questions about the Lancet‘s role in all this – and even whether journals should be publishing papers like this without full and transparent data before there has been adequate regulatory evaluation.
Derek Lowe called some of the developers’ vaccine disinformation “a disgrace”, and I agree. He continued, “What we’re seeing here is a deliberate attempt by the backers of the Sputnik-V vaccine to smear the competition, Pfizer especially. It’s not enough if they succeed – others must fail. This is a vile, destructive tactic…”
It is destructive, but I think it’s about something more than just trying to gain success for this vaccine. Watching all this propaganda pour out over the last few months, and then digging into the analyses of it, I find the argument made in the EU report convincing: there does seem to be a wider geopolitical aim, here, of sowing division and discrediting certain public agencies, not just certain vaccines. And it’s succeeding.
A recent podcast featuring experts in disinformation discussing the Sputnik maneuvering underscores just how much it’s succeeding. They featured this definition of disinformation:
Disinformation is a deliberate falsehood put out to mislead an audience. But what we see more of are true bits of information where necessary context has been removed or manipulated in a way that makes it technically true but wildly misleading.
Bret Schafer, disinformation analyst
Yet even those disinformation experts went on to unquestioningly accept as fact one of the most prominent Sputnik claims that fits that very definition: describing themselves as the first Covid vaccine to get a regulatory green light. The very name, Sputnik V, embodies that claim – as the Sputnik rocket won the space race, so Sputnik V won the Covid vaccine one in August 2020, when it became “The first registered Covid-19 vaccine”, according to their branding and promotion. Except that it wasn’t. China “won” that one – with CanSino, back in June.
And then there’s that now-notorious Twitter account: will Twitter ultimately suspend it because of its stream of vaccine disinformation, because it’s advertising a pharmaceutical product, or both?
Postscript: There have been several further developments in the days since this was written. So here’s a further timeline.
- May 6: Sputnik Light was authorized in Russia – the Ad26 shot as a single-injection vaccination – again without phase 3 trial. It’s based on a calculation of 79% efficacy for the people who failed to get the second shot in the Russian rollout – so it’s a subset of the single-digit percentage of Russians who have used the vaccine, compared with all the unvaccinated people in the country. The likelihood that this group of people differs in behavior and other ways that could affect could also affect their risk of Covid-19 is high. In addition, they cited some immunogenicity data. (More here.)
- The same press release of May 6: They also make the claim, “Sputnik Light has proven effective against all new strains of coronavirus, as demonstrated by the Gamaleya Center during laboratory tests.” As far as I know, none of this is public. The only public data I’ve found on Sputnik (“Light” or full) is a preprint that found 8/12 serum samples from people vaxed with Sputnik V had signs indicating failure to neutralize B.1.351 (“SA” variant), etc.
- May 10 – Slovakia: The tests from Hungary arrived and were reported by the Minister for Health to be “satisfactory”. I could find no statement from ŠÚKL.
- Same day – Mexico: Officials said that Russia was having difficulties producing enough of the second shot, and people who got the first shot probably wouldn’t get the second. Mexico’s assistant health secretary said they had been told the first adenovirus grows more quickly than the second, and the developers had suggested that they give the second shot 6 months later. Sputnik denied the claims. However, Mexico has only received 1.9 million of the 24 million it purchased.
- May 11 – Brazil: Anvisa reported that it filed its response to a political directive to justify its refusal of permission to import Sputnik V to Brazil’s Supreme Court.
- May 12: The Lancet published a letter to the editor by Enrico Bucci and colleagues, detailing the refusals of access to phase 3 trial data and the trial’s protocol. They raised concerns about discrepancies between the trial register entry and the article for the efficacy outcome, and a major shortfall in numbers of participants reported there and in the article, as well as data inconsistencies.
- Same day, May 12: Author reply to the letter to the editor by Bucci was published alongside it, stating that the protocol had been provided to The Lancet (without explaining why it was not published). They say the 13,986 additional participants had either not been randomized or had been excluded according to the trial’s criteria, and reject concerns other than the numerical errors which had been corrected. There is no commitment to provide access to additional data.
- Same day – Slovakia: A report appears to suggest that Sputnik V may be authorized there at the political level as the Minister for Health is satisfied with the documentation available, but I could find no statement from ŠÚKL.
This vaccine is being evaluated for WHO listing. Records in my collection for this vaccine here, including 1 laboratory study of B.1.1.7 (“UK”) and B.1.351 (“Brazil”) variants of concern.
This vaccine also in section on community impact studies.
Sinopharm – Beijing
We finally got a look at a bit of data from its big phase 3 “4 Humanity” trial in the WHO evidence assessment. It’s a 45,000-participant trial, with 15,000 each in groups for the Beijing vaccine, the Wuhan vaccine, and placebo. The results here are for the first 13,765 people getting the Beijing vaccine versus placebo.
People got 2 doses, 21 days apart and vaccine efficacy was:
- Covid-19 in people with no previous infection: 80.8% (CI 67-89);
- Covid-19 in the whole group: 78.1% (CI 65-86);
- Hospitalization: 78.7% (CI 26-94);
- No severe Covid-19 in vaccinated people vs 2 in the placebo group; and
- No Covid-19 deaths in vaccinated people vs 1 in the placebo group.
The trial had very few people over the age of 60 (only 209), and the trial had few women – only 2,167. There were 2 serious adverse events that could possibly be vaccine-related – severe nausea, and a neurological adverse event (inflammatory demyelination syndrome/acute disseminated encephalomyelitis).
In other news for this vaccine:
- The first locally-produced version of the vaccine is being distributed in the UAE – it’s called Hayat-Vax.
- Sinopharm registered a 3-dose trial for people aged from 3 – for signs of immune response and safety, in 4,400 people in China.
This vaccine has been listed by WHO and will be distributed through COVAX. Records in my collection for this vaccine here (check the notes field to see if a record includes the Wuhan vaccine, the Beijing one, or both). Includes 2 laboratory studies on variants of concern, B.1.1.7 (“UK”) and B.1.351 (“SA”).
This vaccine also in: the trials in children section.
Sinopharm – Wuhan
Still no detailed data on this inactivated vaccine (Sinopharm’s WIBP), which has had a scandal-plagued trial stopped for lack of efficacy in Peru (see my last roundup past).
This vaccine is being evaluated for WHO listing. Records in my collection for this vaccine here (check the notes field to see if a record includes the Wuhan vaccine, the Beijing one, or both). No studies on variants on the Wuhan vaccine.
Sinovac CoronaVac
The amount of data on this vaccine grew a lot in the last few weeks, with both community impact studies and more detail on trial results. The WHO released its summary evidence assessment, though we’re still waiting on its final decision about listing the vaccine. They included vaccine efficacy estimates from these studies, with different target groups, definitions of Covid-19 events, differing intervals between first and second doses, and in the context of different variants – again without much data for people over 60:
- Phase 3 trial from Turkey (13,000 people, 14 days between doses): 84% against symptomatic Covid-19 (CI 65-92) and 100% against hospitalization (CI 20-100);
- Study from Chile (10.5 million, included above in community impact, 28 days between doses): 67% against symptomatic Covid-19 (CI 65-69) and 85% against hospitalization (CI 83-87);
- Phase 3 trial from Indonesia (1,620 people, 14 days between doses): 65% against symptomatic Covid-19 (CI 20-85);
- Phase 3 trial from Brazil (12,688 people, 14 days between doses): 51% against symptomatic Covid-19 (CI 36-62) and 100% against hospitalization (CI 56-100); and
- Test-negative case-control study from Brazil (393 case-control pairs, 14 days between doses, included above in community impact): 50% against symptomatic Covid-19 (CI 11-71).
WHO reported that one of the differences in the phase 3 trial in Brazil was a very high level of co-morbidity in participants: 56%. There was data in the WHO presentation and Sinovac’s briefing to them that haven’t been made public before.
In other news:
- The protocol for the phase 3 trial in Turkey was published and a preprint of the Brazilian trial was posted, as well as another of immunogenicity data from the trial in Chile.
- Sinovac released some data on results in children (see section on children above).
- The EMA has begun to review CoronaVac for possible authorization in Europe.
- Sinovac head says they are manufacturing 6 million doses a day and plan technology transfer to enable local manufacture in 10 countries.
- Vaccination was completed in the whole-of-town stepped wedge trial in Serrana – first results expected soon (see community impact section above).
A decision by WHO on listing for distribution in the COVAX scheme is expected shortly.
Records in my collection for this vaccine here. Includes 2 laboratory studies on variants of concern, B.1.1.7 (“UK”), B.1.351 (“SA”), and the P1 (“Brazil”) variant of concern, with some clinical data on P1 in community studies.
This vaccine also in sections on community impact data and children.
Covaxin
Last post we had seen the first interim results for the phase 3 trial of this inactivated vaccine (BBV152), produced by Bharat Biotech and the Indian Council of Medical Research (ICMR). This time, there’s been a second interim result – we’re still waiting for the results when it has reached the target number of Covid-19 illnesses the trial needs.
Efficacy against symptomatic Covid-19 is now at 78% (CI 61-88), based on 127 people who got sick 14 days after the second dose, in a trial 25,800 adults. They report an efficacy rate of 70% against transmission – but it’s not clear how that was established. This is one of the phase 3 trials for which there is no published protocol, and the press release readouts for these interim efficacy results are thin on details. No one in the vaccine group developed severe Covid-19, but the numbers of people hospitalized aren’t reported.
Bharat also announced a phase 2 trial of a third dose, but I haven’t found a trial register entry for that. No word yet on their trial for an intranasal vaccine, called BBV154.
Records for this vaccine in my collection here. Includes 3 laboratory studies on variants of concern, including B.1.1.7 (“UK”), B.1.617 (“Indian”), and P2 variant (one of the “Brazil” line).
Overview of phase 3 trials and what’s new
- There are now 28 vaccines in, or about to be in, phase 3 trials – including 7 additions since the last post: the third of the Cuban protein subunit vaccines, as well as one from Clover (China) and another from Biological E (India). There’s an mRNA from Walvax (China), too. The other 3 are inactivated vaccines from China, France, and Iran.
- Another mRNA vaccine plus Vietnam’s first locally-developed protein subunit vaccine seem to be close behind them.
- The WHO SOLIDARITY for Vaccines Trial comparing multiple vaccines in a shared protocol to a shared placebo group is getting closer – but we still don’t know which vaccines will be included.
- The next 3 major phase 3 trial results expected are for CureVac’s mRNA vaccine, the US/LatAm trial for the Novavax protein subunit vaccine, and Zydus Cadila’s DNA vaccine on trial in India.
The 7 additional vaccines in phase 3 trials underway (or about to be) since my last round-up are:
- Abdala, from the Center for Genetic Engineering and Biotechnology (CIBG) and BioCubaFarma, is a protein subunit vaccine, with 3 doses in the course – boosters at 2 weeks and 4 weeks. It’s the second Cuban vaccine to reach phase 3 trial, and they have already recruited and fully vaccinated 48,000 people in 3 provinces in Cuba. That is blisteringly fast – certainly the fastest large phase 3 Covid vaccine trial. There will also be a trial in Venezuela (no details yet), which will also manufacture the vaccine if it’s successful.
- ArcoV, from the Chinese Academy of Military Science and Walvax (due to start in May) is an mRNA vaccine that doesn’t need super-freezing. The trial is planned for Mexico. A factory is being built to manufacture this vaccine: it’s planned to be ready for mass production in August.
- BECOV2, from Biological E in India, announced approval to run a phase 3 trial in India on April 24, and they hope to be available in August and manufacturing 70 million doses a month. It’s a protein subunit vaccine that was developed by Baylor University in the US. (Biological E is getting $1 billion from the US government to manufacture the J&J vaccine as well.)
- COVIRAN BAREKAT is an inactivated vaccine from Iran, going into phase 3 of its 20,000-participant phase 2/3 trial.
- KCONVAC is another inactivated vaccine from China, this time from Shenzhen Kangtai Biological Products (SZKT). They announced a partnership in Malaysia with YTB Healthcare for 3,000 participants for their phase 3 trial – from a total of 28,000.
- SPECTRA is the name of the phase 2/3 trial for the adjuvanted protein subunit vaccine from Clover Biopharmaceuticals and Dynavax. It’s for 22,000 people in Latin America, Asia, Europe, and Africa. (This is an “on again” trial: Clover had been on the starting blocks with another vaccine developed with GSK, before abandoning it – no reason given – and concentrating on this second candidate with Dynavax.)
- VLA2001, Valneva’s inactivated vaccine, is going into the first superiority trial – that’s a trial designed to show it’s at least as good or better than another authorized vaccine, not placebo. It’s also explicitly based on signs of immunogenicity, not demonstrating efficacy against disease. There will be a non-randomized group of 1,000 people under 30, plus 2,000 randomized to VLA2001 and 1,000 to get the Oxford-AstraZeneca vaccine.
The next vaccines apparently going into phase 3 soon are another mRNA vaccine that doesn’t need super-freezing: from Arcturus, a US company working with a university in Singapore. Plus a phase 3 trial for the first of Vietnam’s locally-developed vaccines, the protein subunit Nanocovax, looks set to start soon.
And the next phase 3 trial results? The international trial for CureVac’s vaccine (the other German mRNA vaccine) may be next: they’re expecting to have their first results by early May. Results should be coming soon, too, from the US/LatAm trial for Novavax, and for Zydus Cadila‘s DNA vaccine, currently on trial in people aged 12 up in India.
The table below is updated, so check out any vaccine you’re interested in. Here are some notable new trials for vaccines that already had phase 3 trials running (or completed):
- Sisonke (Together) trial: When South Africa decided not to use AstraZeneca vaccine, they switched to J&J vaccine. Because it wasn’t yet authorized, they set up a phase 3 trial (but not a randomized one). Details have now been registered: it’s for 500,000 healthcare workers offered the J&J vaccine. They will be studying severe Covid-19 outcomes, and other issues like which variants cause breakthrough infections (getting infected after being vaccinated), and how many healthcare workers agree to get vaccinated.
- Cuba has a trial that seems comparable to the Sisonke trial. It’s for the SOBERANA vaccine, that doesn’t yet have phase 3 results. They will follow 150,000 vaccinated people who hadn’t had Covid-19 previously, to look at their outcomes and study wider issues about the spread of the disease in the community.
- There’s a trial of Moderna vaccine in the US, to study its effect on transmission among university students: for 37,500 students, who will either get vaccinated immediately, or 4 months later.
Addendum: The phase 3 trial tables
Phase 3 trials: recruitment progress
| Vaccine | Target or final size | Single or similar trials? | Reaching target? |
|---|---|---|---|
| Abdala CIBG/ BioCubaFarma Cuba |
48,000 | 1 large trial; further small trial planned | Fully recruited. |
| Ad26.COV2-S Johnson & Johnson USA |
73,783 | 2 large trials | Single-dose trial with 43,783 reported. Second 2-dose trial advanced, perhaps nearing recruitment. |
| Ad5-nCoV CanSino China |
40,500 | Mostly single trial | Press release readout for results of single-dose trial with ca 40,000. |
| ArcoV Walvax/Chinese Academy of Military Science |
28,000 | Single trial | To start in May. |
| BECOV2 Biological E India |
Not yet known | Single trial | Approval announced by company on April 24. |
| BNT162b2 BioNTech/Pfizer Germany |
51,945 | Mostly single trial | Trial with 46,331 aged 16 and over reported; results for additional 2,260 people aged 12 to 15 reported in press release. Trial for 4,000 pregnant women began in February. |
| ChAdOx1 nCov-19/AZD1222 AstraZeneca UK (Oxford Uni) |
54,690 | 3 very different large trials (plus some small) | 2 major trials 10,000+ each (UK and Brazil) reported. Major trial in US and other countries, press release report for 32,449. Small trial in India has final results, but not published. |
| CoVLP Medicago Canada |
30,918 | 1 trial | Began in March. |
| CVnCoV CureVac Germany |
41,140 | 1 large, several small | Large international trial fully recruited. |
| EpiVacCorona VECTOR Russia |
3,000 | 1 trial | Apparently fully recruited. |
| GrAd-CoV-2 ReiThera Italy |
10,300 | 1 trial | Phase 2/3 began February/March. |
| mRNA-1273 Moderna USA (NIH) |
40,170 | 1 large, 3 small |
Trial of 30,420 reported. Trial of 3,000 adolescents reached full recruitment in March. (Trial for booster modified for variant began in March, and so did a trial in infants and children.) |
| NVX-CoV2373 Novavax USA |
46,600 | 3 trials |
Trial of 15,000 in UK fully recruited. US/Mexico trial of 30,000 fully recruited. (Efficacy results also from 4,442 in a phase 2b trial in South Africa.) Small bridging trial in India began in March. |
| SCB-2019 Clover/ Dynavax China/USA |
22,000 | 1 trial | Trial began in March. |
| SOBERANA-2 Finlay Institute Cuba |
44,010 | 1 trial | Began in March. Unknown number also soon in Iran. |
| Sputnik V, Sputnik Light (Gam-COVID-Vac) Gamaleya Russia |
41,700 | Mostly single trial. Small trial of Sputnik Light. |
Trial in Russia stopped recruiting at 31,000. Small trial in UAE fully recruited; small trial in India has completed dosing. Sputnik Light trial began in February. |
| UB-612 COVAXX USA |
7,320 | Single trial | Phase 2 of 2/3 trial, start date not known. |
| ZF2001 Chinese Academy of Sciences |
29,000 | Single trial | Started in November. |
| ZyCov-D5 Zydus Cadila India |
30,000 | Single trial | Fully recruited. |
| Inactivated virus vaccines | |||
| BBIBP & WIBP Sinopharm China |
60,300+ | 2 large trials, several small |
Trial with 45,000 fully recruited. Trial with 12,000 fully recruited in Peru. |
| CoronaVac Sinovac China |
31,020+ | 2 large trials, several small, 1 unknown |
Trial with 13,060 healthcare workers in Brazil fully recruited. |
| Covaxin Bharat Biotech, Indian Medical Research Council India |
29,500+ | Mostly a single trial | Trial efficacy readout for 25,800 participants. |
| COVIRAN BAREKAT Shifa-Pharmed Iran |
20,000 | Single trial | Started in April. |
| KCONVAC Shenzhen Kangtai Bio China |
28,000 | Single trial | Beginning in May? |
| QazVac Scientific Research Institute Kazakhstan |
3,000 | Single trial | Fully recruited, results expected in April. |
| (Unnamed) Chinese Academy of Sciences |
34,020 | 1 trial | Began in January. |
|
VLA2001 |
4,000 | Single trial | Superiority immunogenicity trial, VLA2001 vs ChadOx1. Started in April. |
| Total participants |
Over 852,000 |
About 600,000 people likely to be already recruited. |
The phase 3 trials, and the preclinical and clinical trial results reported for those vaccines
| Vaccine Company Country |
Preclinical | Phase 1 results People |
Phase 1/2 results People |
Phase 2 results People |
Phase 3 Total people |
| Abdala CIBG/ BioCubaFarma Cuba |
Unpublished | n.a. |
Unpublished (132) |
n.a. |
48,000 (Cuba) (Trial planned in Venezuela) |
| Ad26.COV2-S Johnson & Johnson USA |
Primate, non-primate | n.a. | 1,045 |
n.a. (Trial for 824 pregnant woman to start) |
73,783: 43,783 (single shot) (Argentina, Brazil, Chile, Colombia, Mexico, Peru, South Africa, USA) Results reported 30,000 (2 shots) (Belgium, Colombia, France, Germany, Philippines, South Africa, Spain, UK, USA) |
| Ad5-nCoV CanSino China |
Non-primate only | 108 | n.a. |
508 (Plus unreported trial in children) |
40,500: 40,000 500 |
| ArcoV Walvax/Chinese Academy of Military Science |
Non-primate only | Unpublished (168) | n.a. |
Unpublished (420) |
28,000 (Mexico) |
| BECOV2 Biological E India |
Non-primate only | n.a. | Unpublished (360) | n.a. | (Seeking approval in India) |
| BNT162b1-b3 BioNTech/Pfizer Germany |
Primate, non-primate (b2 only) | b1:
45 b1: 60 b1 & b2: 195 |
n.a. |
(Phase 2/3 trial) (Phase 2 for people with allergies underway: 3,400, includes Moderna) (Bridging trials in China & Japan) |
51,945 46,331 (b2)* 4,000 (b2)** (USA) (pregnancy) 1,530 (b2) (USA) (consistency) 84 (lyophilized b2) (USA) |
| ChAdOx1 nCov-19/AZD1222 AstraZeneca UK (Oxford Uni) |
Primate, non-primate | n.a. | 1,077 |
560 (from the major phase 2/3 trial) 2,026 (from South Africa, HIV-negative) 300 (Phase 2 trial underway for ages 6 to 17) |
54,690: 12,390** 30,000 10,300 1,600** 400 –100***– |
|
CoVLP |
Unpublished | 180 | n.a. | Unpublished (918) | |
|
CVnCoV |
Primate, non-primate | 247 | n.a. | (Phase 2/3 trial) |
41,400 36,500** 2,520 1,000 (with flu vax) (Argentina, Colombia, Peru) 180 (comparing older people to younger) (Not stated) 1,200 (people with co-morbidities) (Not stated) |
|
EpiVacCorona |
Unpublished | 14 | n.a. | 86 |
3,000 |
|
GrAd-CoV-2 |
Primate, non-primate | 90 | n.a. | (Phase 2/3 trial) |
10,300** |
| mRNA-1273 Moderna/NIH USA |
Primate, non-primate |
85 (Phase 1 for variant modification underway: 210) |
n.a. |
600 (Phase 2 for people with allergies underway: 3,400, includes BNT-Pfizer) |
40,170 30,420 adults 3,000 young people** 6,750 infants and children** |
| NVX-CoV2373 Novavax USA |
Primate, non-primate | 131 | n.a. |
1,288 (Australia, USA) 4,387 (South Africa) |
45,000: 15,000 30,000 Small bridging trial planned in India |
| SCB-2019 Clover/Dynavax China/USA |
Primate, non-primate | 148 | n.a. |
(Phase 2/3 trial) |
22,000** (Brazil, Philippines – South African & European sites planned) |
|
SOBERANA-2 (Finlay-Fr-2)/SOBERANA-1 (Finlay-Fr-1) |
2: Non-primate 1: Unpublished |
2: Unpublished (40) 1: 30 (Unpublished studies for 676 & 60) |
n.a. |
2: Unpublished (910) |
44,010 |
|
Sputnik V & Sputnik Light (Gam-COVID-Vac) |
Unpublished | n.a. |
76 Sputnik Light: (Phase 1/2 trial underway: 110) |
n.a. |
41,700 31,000 100 2,000 1,600** 1,000 6,000 – Sputnik Light (Russia) |
|
UB-612 |
Non-primate | Unpublished (60) | n.a. | n.a. (phase 2/3) |
7,320** |
|
ZF2001 |
Primate, non-primate |
50 (Unpublished, 50 aged 60+) |
n.a. | 900 |
29,000 |
|
ZyCov-D |
Primate, non-primate | n.a. | Unpublished (1,048) | n.a. |
30,000 |
| Inactivated vaccines: | |||||
| BBIBP & WIBP (Beijing & Wuhan) Sinopharm China |
Primate, non-primate (Wuhan) Unpublished |
n.a. | 320 (Wuhan)
640 |
n.a. |
60,300+: 45,000 (both vaccines) 3,000 (Beijing only) 300 (Wuhan only) Apparently additional: 12,000 (both vaccines) (originally 6,000) ? (both vaccines) ? (Beijing only) |
| CoronaVac (PicoVacc) Sinovac China |
Primate, non-primate | 239 | Phase 1/2 trial for ages 3 to 17 underway | 950 |
31,020+: 13,060 (originally 9,000) 13,000 1,620 Unknown – for people 60+ 2,300 1,040 (non-inferiority study of vaccine lots) |
| Covaxin (BBV152) Bharat Biotech/Indian Medical Research Council India |
Non-primate | 375 | n.a. | 380 |
29,500-30,500: 28,500 (India) Efficacy readout in press release 1,000-2,000 (planned, Bangladesh) |
| COVIRAN BAREKAT Shifa-Pharmed Iran |
Non-primate | Unpublished (88) | n.a. | n.a. (Phase 2/3) |
20,000** (Iran) |
| KCONVAC Shenzhen Kangtai Bio (China) |
Unpublished | 60 | n.a. | 500 |
28,000 (Malaysia, others unknown) |
| Qaz-Covid-in Scientific Research Institute Kazakhstan |
Unpublished | n.a. | Unpublished (244) | n.a. |
3,000 (Kazakhstan) |
| Unnamed Chinese Academy of Medical Sciences |
Non-primate | Unpublished: 200 (aged 18 to 59) | Unpublished: 471 (aged 60+) | 742 (aged 18 to 59) |
34,020 (Brazil, Malaysia) |
| VLA2001 Valneva France |
Unpublished | n.a. | Press release only (153) | n.a. |
4,000 (UK) (VLA2001 vs ChadOx1) |
* a combined phase 1/2/3 trial
** a combined phase 2/3 trial
*** an unrandomized phase 3 trial
n.a. = not applicable
Sources: unless otherwise linked, the sources are from my tagged public Zotero collection (detailed below)
~~~~

All my Absolutely Maybe Covid-19 vaccine posts
All previous Covid-19 posts at Absolutely Maybe
My posts at The Atlantic, at WIRED, and debunking posts at my personal website.
[Correction May 14] A para describing a study in the UK in the community impact section was deleted: it had already been included in the previous post, and was selected again when a further preprint was posted.
[Corrections May 15] Corrected typos notified by JZH and Lieven Vandelanotte. (Thanks, JZH and Lieven!)
[Correction May 17] I had failed to finish the section on Moderna’s M1a results – sentence added.
[May 20] Updated KCONVAC phase 3 trial size with trial register entry.
Disclosures: My interest in Covid-19 vaccine trials is as a person worried about the virus, as one of my sons is immunocompromised: I have no financial or professional interest in the vaccines. I have worked for an institute of the NIH in the past, but not the one working on vaccines (NIAID). More about me.
The cartoons and infographics are my own (CC BY-NC-ND license). (More cartoons at Statistically Funny.)
Infographic notes:
The infographic scale was determined by 95% as the highest established efficacy rate with 50% as the minimum set by WHO and the US FDA, enabling a breakdown into equal-sized low-medium-high areas: data shown in table. The low-medium-high scale for rates of adverse events was set with the rate for the inactivated vaccines as lowest established rate, with the highest rate just above the highest for the most common systemic adverse event any of these vaccines at the other end (75%), after taking both the rate of the most commonly reported severe outcome and the age of the trial population into account.
Sources for study records
Source records are in my public Zotero collection of Covid-19 vaccines that have any published preclinical or clinical results (or preprints), or that are in phase 3 trial (more details below, including how to use it) for general populations. It also includes the matched control studies I am featuring. Please let me know if I’m missing any! On May 13, the collection included 583 entries:
- 149 vaccines/groups with published or posted results;
- 23 trial protocols, for 8 vaccines and Com-COV (including 1 vaccine with additional protocol versions in a clinical study report package);
- 190 preclinical preprints/articles;
- 57 preclinical or serum studies of vaccines and new variants;
- 173 trial registry entries associated with these vaccines;*
- 64 clinical trial preprints*/articles/letters:
- 24 for phase 1 trials;
- 14 for phase 1/2 trials;
- 11 for phase 2 trials;
- 2 combined reports of phase 2 and 3 trials;
- 1 combined report of phase 1 to 3 trials;
- 6 phase 3 trials (for 5 vaccines);
- 3 author replies to letters to the editor about their publications;
- 3 errata notices for 2 vaccines;
- 29 trial efficacy readouts (press releases or website), for 13 vaccines;
- 29 documents reporting on or reviewing phase 3 data at regulatory agencies, for 8 vaccines;
- 2 full clinical study report packages;
- 3 termination notices, for 4 vaccines (all but one of which have other records in this collection);
- 11 featured community impact studies, for 5 vaccines;
- Records for 5 vaccines tagged as trials including children/teenagers;
- Records for 2 vaccines tagged as adapted for variant;
- Record for 1 vaccine tagged as geographic.
* May not include all entries where a trial is registered in multiple registers, or a preprint is posted to multiple servers.
Notes on the collection
This is a publicly accessible collection I update regularly. It includes any Covid-19 vaccine with published preclinical, clinical trial results, or trial protocols. If a phase 3 trial starts (or is about to) without any prior publications, that trial would also be included. Once a vaccine is in the collection, clinical trial register entries for that vaccine are also added.
When trials are registered in more than clinical trials registry, the multiple records may or may not be in the collection: If I have located a record in ClinicalTrials.gov, I do not hunt for additional registrations. For my own convenience in keeping an overview of vaccine progress, when preprints appear later in journals, I over-write the original record with the journal article. Preprints may also be uploaded to multiple preprint servers: rather than check if versions have differed, I keep the first preprint in the collection (sometimes over-written with updates in the same server), unless I saw that a version that seems markedly different.