This month, Arcturus’ self-amplifying mRNA vaccine was approved for use in the UK. There were also new clinical trial results for another…
A Big Hit, A Big Miss, & Mixed Vaccine Schedules: The 18-Month Mark

Remember when we were told the earliest we could possibly hope for a vaccine was 12 to 18 months? Here we are, coming up on 18 months after the sequencing of the SARS-CoV-2 genome: people are calling vaccines with delivering results now “late”! And 15 vaccines have at least interim phase 3 results.
While this was all going on, a group of documentary-makers were interviewing people in real time to tell the story as history unfolded. The results is called The Vaccine, and I hear it’s fantastic – try to catch it if it’s broadcast in your country. (Disclosure: I was one of the people offering advice behind the scenes.)
Another thing we were being told early in the pandemic: that SARS-CoV-2 didn’t seem to mutate quickly and we didn’t have to worry about variants. If only that had been true! Now there are so many to worry about, WHO is giving them code names from the Greek alphabet – they’re keeping track of these new names here.
Something else we hoped wouldn’t happen, did: there are very large rich countries that have vaccinated more than half their population – with one even taking vaccines from COVAX – but less than 2% of Africa’s 1.3 billion people have even a single dose. Facing fearsome outbreaks, some have rolled out locally-developed vaccines before phase 3 trials results are in.
Which brings us to the big hit and the big miss in this post’s title. The first is the over 90% efficacy results for the Novavax US/Mexico trial. Similarly high efficacy was reported for another protein subunit vaccine, Cuba’s 3-dose Abdala vaccine, but there’s not enough data to get excited just yet. The big miss was CureVac’s mRNA vaccine which didn’t reach the minimum for Covid vaccine efficacy. And there were results in between those highs and that low for a 2-dose course of Cuba’s conjugate vaccine, Soberana, which is also being tested in 3 doses – and the first results for a Covid DNA vaccine, India’s ZyCov-D. You can read more about these in their separate sections below (see the contents list). (I also wrote about the Novavax vaccine and mRNA hype at The Atlantic.)
We still need more vaccines, and more data on all the vaccines – especially how long immunity lasts, for example. We are extremely lucky that there are now well around 600,000 people in phase 3 trials for Covid vaccines, building an enormous base of critical information. A big thank you to everyone participating! That number could get close to a million in the coming months, and trials are shifting into under-vaccinated countries. Pakistan appears to be the first country to say no more placebo-controlled trials on ethical grounds – although reportedly, companies aren’t willing to do head-to-head trials against each other. There are reportedly 3 that are willing, though, to participate in the WHO’s SOLIDARITY trial for vaccines: it could be starting soon in the Philippines, and perhaps Colombia, too. There will be a single shared placebo group. No word on which 3 vaccines.
Because there’s so much to unpack in this post, lengthy sections start with a summary of key points at the top – and there’s a contents list with links so you can navigate around. But first some explanations for heavily used terminology in this post.
Key terms used in this post:
- Vaccine efficacy is a rate of risk reduction in symptomatic Covid-19 unless otherwise specified. Vaccine efficacy of 80% or 90% means if a vaccinated person is exposed to the virus, their risk of getting the disease is lowered by that proportion, so it depends on how high their risk of being exposed, and that varies. It’s not an absolute drop in percentage points. (Efficacy is for results from phase 3 clinical trials; effectiveness studies follow that.)
- When available, a range of statistical certainty for efficacy is shown, eg 92% (CI: 88-95). The distance between 88% and 95% in this example is small: it means there is a lot of certainty that 92% is about what we can expect. However, the wider that range is, the more uncertain we are.
- The rate of efficacy set for whether a Covid vaccine works well enough is 50% (with a CI starting at 30% at least).
- And I have a post explaining the terms used, and assessment processes, for adverse events and safety in these trials – including the difference between “severe” and “serious”.
Contents
- Mixed vaccine schedules
- Community impact studies
- Study results in people with compromised immune systems
- The landscape: 11 vaccines with enough phase 3 results
- What else is new for the 15 vaccines with results
- Overview of phase 3 trials and what’s new
- Addendum: phase 3 trial recruitment status and studies
- Sources for study records and notes on using the collection
Mixed vaccine schedules
- There are now early reports of mixed vaccine schedules from 2 clinical trials plus one in animals, as well as the Sputnik V and Soberana trials. There are also 6 reports of results from observational studies.
- The overall results so far are based on viral vector vaccines (mostly AZ) with an mRNA vaccine (mostly BNT-Pfizer). This evidence suggests that mixed schedules may provide a greater immune response than a same-same vaccine course – with the same or higher rate of adverse reactions. It’s possible interval between doses may affect the rate of adverse events, but there is no data from a trial on this yet. It’s not yet clear, though, whether a mixed vaccine schedule is superior to same-same BNT-Pfizer.
- There are 19 clinical trials comparing mixed schedules underway or planned, in 17 countries, involving 22 vaccines (some of them still in the early stage of research). [Update July 3: Plus a study planned in Argentina.]
It’s called heterologous prime-boost vaccination: it’s where instead of first (“prime”) and second (“boost”) injection of the same vaccine, they’re 2 different ones – or perhaps a second boost (third shot) is another vaccine. The theory is that this could widen immune responses more than same-same vaccination, as vaccines are targeted at stimulating the immune system in different ways.
Counting full-course Sputnik V, which is a 2-vaccine combination, and Soberana-02 with a third dose of Soberana-Plus, there are now combinations of 21 vaccines being studied. Some of the vaccines are still experimental. Along with the main Sputnik V and Soberana trials, they’re listed in chronological order in the table below from when they were made public. Since my last post, there are 10 new trials of combinations (marked *).
There are some early clinical trial results from comparisons – COM-COV in the UK (here and here), and CombiVacS in Spain. COM-COV is a randomized trial testing of 4 vaccines (AZ, BNT-Pfizer, Moderna, and Novavax), alone and in combinations – and each at either 4 or 12 week intervals. There were 463 people in the first reports of results. CombiVacS was a randomized trial set up when AZ wasn’t being recommended locally, to assess immune response and short-term adverse events after a follow-up dose of BNT-Pfizer vaccine 8-12 weeks after a shot of AZ: 450 people got the vaccine, and 226 had no second injection. There was also a trial of mixed vaccine schedules in animals (AZ and Covac1, the mRNA vaccine from Imperial College).
COM-COV has released adverse events data and immune response data, but only for the 4-week interval, and only for AZ and BNT-Pfizer. The preclinical study found higher signs of immunity from a mixed vaccine schedule than from either same-same vaccine course; and in CombiVacS, signs of immune response were higher than was reported in the trials of same-same AZ vax.
The 2 trials in people show you get at least the adverse reactions from each vaccine. In the case of the AZ vaccine followed by an mRNA, this can be a high rate overall, as the AZ vaccine’s first shot is the one with the most adverse reactions, and it’s the second shot of mRNA vaccine that has the most adverse events. In COM-COV, where the 2 injections were closer together than CombiVacS, the adverse events after the 2nd injection were higher than from either same-same vaccine alone. (The adverse reaction rate for Sputnik V seems to be at the high end as well.)
There are also 6 observational studies, reporting on outcomes where mixed vaccine schedules have been rolled out, but those who got a particular vaccine first could be at different risk to vaccines introduced later, and the interval between vaccines isn’t as standardized as it would be in a trial:
- A prospective observational study in 340 hospital workers in Berlin: AZ then BNT-Pfizer from 10-12 weeks later had a slightly better immune response than same-same BNT-Pfizer 3 weeks apart, and the mixed schedule had a higher rate of adverse reactions than same-same BNT-Pfizer.
- Another report from Germany (Saarland), with 97 people who had AZ then BNT-Pfizer or Moderna, 55 same-same AZ, and 64 same-same mRNA. AZ then mRNA had similar immune response to same-same mRNA, and both were higher than same-same AZ. AZ-mRNA had the highest rate of adverse events.
- A report for 87 healthcare workers in Hannover (Germany) who had AZ vaccine and either AZ (32 people) or BNT-Pfizer boost (55 people). They also measured signs of immunity in a group of 46 who had 2 doses of BNT-Pfizer. Signs of immune response increased substantially more in those receiving the BNT-Pfizer boost after AZ, especially for variants of concern, and were similar to those having 2 doses of BNT-Pfizer.
- Report for 26 people in Ulm (Germany), who had AZ first then BNT-Pfizer 8 weeks later: higher immune response than people who had same-same, including in lab tests for variants. Adverse events not higher than expected.
- A report from Italy of 8 people in the GrAd-CoV-2 phase 1 trial (gorilla adenovirus-based vaccine), who had 1 shot of that vaccine, and then got either BNT-Pfizer or AZ vaccine.
- A “proof of concept” report of 2 people who had a first dose of an inactivated vaccine (the unnamed one from IMBCAMS/Chinese Academy of Medical Science) followed by the mRNA vaccine from Stemirna Therapeutics in China 7 months later. Signs of immunity were boosted. (Records in my collection on the IMBCAMS vaccine here – it’s in phase 3 trial, and the Stemirna vaccine here – it’s in phase 1.)
A study is reportedly planned in South Korea, too, of mixing doses after AZ vax. Here are the trials I know of, including the clinical trials and the animal study:
| Vaccines | Schedule | Participants | Location(s) | Research phase |
| Sputnik V: rAd26-S & rAd5-S | rAd26-S first; placebo control | 19,866 | Russia | Phase 3 trial, results. |
| Oxford-AZ; Sputnik Ad26 | Alternating | 100 people | Belarus, Russia | In phase 1/2 trial. |
| Oxford-AZ; Sputnik Ad26 | Alternating | 100 people | Azerbaijan | In phase 1/2 trial. |
| Soberana02; Soberana-Plus | 2 doses Soberana02, 3rd dose Soberana-Plus | 44,010 | Cuba | Phase 3 trial. |
| Oxford-AZ; Sputnik Ad26 | Alternating | 100 people | Not specified (near Azerbaijan) | In phase 1/2 trial. |
| Oxford-AZ; Imperial College saRNA | Single shots; alternating combination; usual 2-shot course | 6 groups; 22 mice each | UK | Preclinical, results. Combos had highest response, followed by usual Imperial College course. |
| Oxford-AZ; BNT-Pfizer; Moderna; Novavax | Alternate boost starting with AZ or Pfizer (8 or 12 weeks apart) | 1,050 people | UK | In phase 2 trial called Com-Cov. First results; second report. |
| ChAdV68-S; ChAdV68-S-TCE; SAM-LNP-S; SAM-LNP-S-TCE (ChAd = chimp adenovirus, SAM = self-amplifying mRNA/saRNA) | ChAds first then SAMs; SAMs first then placebo | 140 people | USA | In phase 1 trial. |
| CanSino; ZF2001 (protein subunit) | CanSino followed by ZF2001 or a flu vax as a control group | 120 people | China | In phase 4 trial. |
| Oxford-AZ; BNT-Pfizer | AZ followed by Pfizer | 600 people | Spain | In phase 2 trial called CombiVacS. First results. |
| Oxford-AZ; BNT-Pfizer; Moderna; (?) Sputnik | Alternate boost starting with AZ | 600 people | Italy | Trial not yet underway. |
| *BNT-Pfizer; Sinovac’s CoronaVac | BNT-Pfizer followed by CoronaVac | 100 people | Hong Kong | Media interview only. People who want to switch because of reaction to BNT-Pfizer vaccine. |
| *Oxford-AZ; BNT-Pfizer; Novavax; Moderna; Valneva; CureVac; J&J | Single dose of Oxford-AZ or BNT-Pfizer followed by the same or another Covid vax (or MENACWY control), some in different doses | 2,886 people | UK | Phase 2 trial called COV-BOOST, start was planned for June 1. |
| *CanSino; Sinovac’s CoronaVac | Alternate boost 3-6 months after 1 or 2 doses of CoronaVac | 300 people | China | Phase 4 trial, start was planned for May 25. |
| *Oxford-AZ; BNT-Pfizer; Moderna | Same-same & alternating boost for mRNAs, 4 or 16 weeks apart; AZ first then mRNA boost, 4 or 16 weeks apart | 1,200 people | Canada | Phase 2 trial, called MOSAIC, started on May 20. |
| *BNT-Pfizer; Moderna | Alternating | 400 people | France | Trial called ARNCOMBI, started on May 28. |
| *Oxford-AZ; BNT-Pfizer | Same-same (BNT-Pfizer 3-7 weeks apart, Oxford-AZ 12 weeks apart); Oxford-AZ followed by BNT-Pfizer (12 weeks apart) | 3,000 people | Austria | Phase 2 trial, called HeVACC, started on May 10. |
| *Oxford-AZ; BNT-Pfizer; Moderna; Sputnik V; Sinovac CoronaVac; Covaxin | Not known | 3,000 people | Philippines | Announcement only available. |
| *J&J; BNT-Pfizer; ImmunityBio | ImmunityBio in various forms (eg nasal spray) as a booster | South Africa | Press release only. | |
| *J&J; BNT-Pfizer; Moderna | J&J single shot, or J&J followed by J&J or mRNA | 432 people | Netherlands | Trial called SWITCH, due to start on July 15. |
| *IMBCAMS; Stemirna | IMBCAMS, 2 doses (inactivated) followed by either IMBCAMS or Stemirna (mRNA) 6 months later | 112 people | China | Unrandomized trial. |
Community impact studies
- Multiple controlled community studies confirm the high effectiveness of BNT-Pfizer vaccine against symptomatic Covid-19, with reduced but still high effectiveness against several variants of concern.
- There is evidence from community studies that the BNT-Pfizer and Moderna vaccines reduce asymptomatic and symptomatic disease, severe disease, hospitalization, death, severity of symptoms, and viral loads.
- There is evidence from community studies that AZ vaccine reduces symptomatic Covid-19, severe disease, and hospitalization, including against variants of concern, although it might be struggling with preventing disease caused by the Delta variant.
- Some community data from the UK suggests the Oxford-AstraZeneca vaccine, with second dose delayed, might increase in effectiveness over a month to 70% or more (as it did in exploratory trial analyses here and here).
- Some controlled evidence confirms the community effectiveness of J&J vaccine against symptomatic Covid-19, including where infection caused by variants of concern is common.
- There is some evidence that Sinovac’s CoronaVac offers protection against Covid-19 and hospitalization, including in Gamma outbreaks – in those outbreaks, effectiveness may be reduced, particularly in the very old.
- There is some evidence that the BNT-Pfizer and AZ vaccines reduce infection in unvaccinated household members of the vaccinated.
Once you start studying vaccine rollouts, you have to struggle with a lot of “noise” in what’s sometimes called “real world data”: you often can’t even being to untangle the effects of differences in who gets vaccinated first, for example, or the effects of community-wide actions like lockdowns. (I discussed that a bit more when I introduced this section.)
So I concentrate on community impact studies that have used a design that reduces the biases (such as matched controls for effectiveness studies), and done in very broad groups of people or geographic regions. I tag these studies in my records collection. The goal here is to maintain a rough picture of what we know that either confirms (or not) that the results of the randomized trials apply to the broad community; or that addresses questions the trials could not. The summary points above have been accumulating from all the community impact studies I’ve featured across recent posts.
But before we get to the new featured group and an update on “whole-of-town” studies, here are some other studies with less-controlled designs that I think add something of note in areas not yet covered by enough rigorous studies.
- National data for Uruguay for up to May 25. The data were raw, without controlling for critical factors like the most vulnerable people having been vaccinated first with BNT-Pfizer vaccine. At that point, 46% of the population had at least 1 dose, mostly Sinovac’s CoronaVac. (See my Twitter thread on this.) Out of over 700,000 who had been fully vaccinated with CoronaVac for at least 14 days, only 19 needed intensive care and 6 died (effectiveness against intensive care 95%); for 150,000 with BNT-Pfizer, only 1 needed intensive care and 8 died, all aged 80 or over (effectiveness against intensive care 99%).
- A study of 23 healthcare workers from an Italian hospital who had breakthrough infections after 1 dose of BNT-Pfizer found it reduced symptoms, the duration of symptoms, and viral clearance.
- In a hospital in the Republic of Korea (South Korea), a study of healthcare workers getting the BNT-Pfizer and AZ vaccines found that adverse reactions were higher for AZ vaccines, and for the under 40s it was more likely to result in medical care and an impact on work productivity (impaired performance or absence from work).
- A study linked positive test results in people with the same home address in England to vaccination records. The rate of other people in the house testing positive for SARS-CoV-2 was 10% for the unvaccinated, and about 6% for those who had been vaccinated with BNT-Pfizer or AZ vaccine – at that point, it was mostly a single dose.
- A study of clusters of breakthrough infections in over 100 healthcare workers at 3 Indian hospitals during the Delta outbreak concluded people fully vaccinated with AZ vaccine were being infected and transmitting the disease. Laboratory tests on antibodies suggested that the Delta strain was more likely to be able to evade the AZ vaccine than the BNT-Pfizer vax.
- A study of over 80,000 residents of 280 nursing homes in the US suggested that a high level of BNT-Pfizer or Moderna vaccination in staff and residents, with continued use of masks and other measures protected unvaccinated residents. By my calculation, about 40% of residents were living in homes where less than 59% of the staff were vaccinated.
I’ve added 10 new studies, bringing the total featured across my posts to 23 – the summary points for this section cover all 23. They include BNT-Pfizer, Moderna, and AZ, with 1 each on the J&J vax and CoronaVac. (In 1 of the points below, there are 2 studies.)
An explanation of my search strategy for studies is at the end of this section. And I look at studies on immunosuppressed people separately below.
- USA – BNT-Pfizer and Moderna vaccines were highly effective while Alpha was becoming the dominant strain
- USA – Effectiveness of BNT-Pfizer and Moderna vaccine against hospitalization was estimated at 89%, and 99% against death
- USA – Effectiveness of full BNT-Pfizer and Moderna vaccination against asymptomatic or symptomatic SARS-CoV-2 infection was 91%, and breakthrough infections were milder with lower viral loads
- USA – Only 1% of people admitted to hospital for Covid-19 were fully vaccinated with BNT-Pfizer and Moderna, generally older people with major health problems
- Canada – The effectiveness of single shot of BNT-Pfizer or Moderna vaccine in people aged 70 and older was around 70% against Alpha and non-variant strains, lower against Gamma
- Canada – Vaccine effectiveness after 2 doses of BNT-Pfizer or Moderna vaccine was close to complete for severe disease (caused mostly by the Alpha variant)
- UK – People were more likely to get infected after vaccination if they were frail and elderly, or lived in a deprived area; fewer symptoms were reported for breakthrough infections than infections in unvaccinated people
- UK – BNT-Pfizer was very effective against hospitalization and symptomatic disease caused by the Delta variant after 2 doses; AZ was very effective against hospitalization, but substantially less against symptomatic disease
- Brazil – Sinovac’s CoronaVac remained effective against symptomatic disease after 2 doses in a Gamma outbreak in people aged 70-74, but not older; no data on protection from severe outcomes
- First results in whole-of-town studies
USA – BNT-Pfizer and Moderna vaccines were highly effective while Alpha was becoming the dominant strain
This is a major study in healthcare workers across the US, based on their Covid test results. It includes data up to March 18. Alpha was the dominant variant at that point: it had spreading in the US from January. There were 623 “cases” and 1,220 controls. Vaccination had mostly been with BNT-Pfizer (about 80%), the rest with Moderna vax.
It was mostly healthcare workers who had direct-patient contact who were getting tested: most were aged 19-49, female, non-Hispanic white, and had conditions that would put them at risk if they got Covid-19. After adjusting for age, race, and underlying conditions, vaccine effectiveness from 14 days after dose 1 was 81.7% (CI 74-87), and from 7 days after dose 1 it was 93.5% (CI 87-97).
Test-negative case-control study – Pilishvili 2021 – Protocol for this study here.
USA – Effectiveness of BNT-Pfizer and Moderna vaccine against hospitalization was estimated at 89%, and 99% against death
This is a study done in the Veterans Health Administration testing service, up to the end of February (before Alpha had become dominant in the US). For the analysis of hospitalization and death, people who tested positive (cases) were matched on geographic region and approximate testing date to up to 4 people who tested negative (controls). They were able to match 15,404 cases with 61,610 controls.
There were 2 studies – a test-negative study on vaccine effectiveness of 1 and 2 doses of either BNT-Pfizer or Moderna: that study found a rate of effectiveness similar to the trials (94% after full vaccination, CI 92-95).
The second case-control study analyzed vaccine effectiveness for hospitalization and death:
- Partial vaccination: 40% against hospitalization (CI 27-50), and 54% against death (CI 21-74);
- Full vaccination: 89% against hospitalization (CI 81-93), and 98.5% against death (CI 87-99.8).
Test-negative case-control (for infections) and case-control study (for hospitalization and death) – Young-Xu 2021 (preprint)
USA – Effectiveness of full BNT-Pfizer and Moderna vaccination against asymptomatic or symptomatic SARS-CoV-2 infection was 91%, and breakthrough infections were milder with lower viral loads
This is a prospective study of 3,975 healthcare, essential, and frontline workers who volunteered to get weekly Covid tests from late December, as vaccination was being rolled out in the US. Data on the experience of vaccinated and unvaccinated through to April 10 was analyzed using propensity weighting for being vaccinated, study site, occupation, and daily records of local viral circulation (how many people were testing positive in that general community). (Propensity weighting is one of the techniques used to try to control for bias in studies that aren’t randomized trials.)
There were 204 SARS-CoV-2 infections detected in that time: 5% of the participants overall, mostly when they were unvaccinated (156 people), with 11 people infected who were partially vaccinated with either BNT-Pfizer or Moderna vax, and 5 breakthrough infections in fully vaccinated people (14 days or more after dose 2). Data on symptoms for those people came from surveying them when they got their result and later, as well as medical records. (There were 33 people who were vaccinated with the J&J vaccine, but because the number was so small, they weren’t included in this analysis.)
Vaccine effectiveness against asymptomatic and symptomatic Covid-19 was 91% for the fully vaccinated (CI 76-97) and 81% for the partially vaccinated (CI 16-57). When people were infected, if they were partially or fully vaccinated their viral load was 40% lower than unvaccinated people (CI 16-57) – which could reduce transmission of infection. Their risk of symptoms with fever was less than half (relative risk of 0.42, CI 0.18 to 0.98), and they spent 2.3 days less in bed (CI 0.8-3.7).
Prospective study with weekly testing, and propensity weighting – Thompson 2021 (with protocol)
USA – Only 1% of people admitted to hospital for Covid-19 were fully vaccinated with BNT-Pfizer and Moderna, generally older people with major health problems
This study is from an 8-hospital healthcare system in Michigan, from December to the end of April. Alpha was the dominant variant at that point: it had spreading in the US from January. The vaccines being used at that time were BNT-Pfizer and Moderna.
Out of over 11,800 people hospitalized for Covid-19 in that time, only 129 were fully vaccinated, and 825 were partially vaccinated. The fully vaccinated people in that 1% of hospital admissions were generally aged 65 or over and had health problems: the researchers classified them as at “high-risk for near term death” (including all 8 deaths and 6 people who needed ventilation).
The researchers analyzed a group of 387 people using propensity matching: that suggested that full vaccination reduced risk of a combination of severe disease outcomes – though it didn’t reach statistical significance) – and partial vaccination did not.
Propensity-matched case-control study – Bahl 2021 (preprint)
Canada – The effectiveness of single shot of BNT-Pfizer or Moderna vaccine in people aged 70 and older was around 70% against Alpha and non-variant strains, lower against Gamma
This was a study in people aged 70 and over getting tested for Covid-19. The researchers analyzed 16,993 test results: 1,226 were positive (7.2%). The strains were known for 92% of the infections – about 45% were Alpha and 28% were Gamma. About 12,000 of the people had a single dose of vaccine; 85% BNT-Pfizer, and the rest, Moderna.
From 21 days after the single shot, vaccine effectiveness against strains that weren’t variants of concern was 72% (CI 58-81). Against Alpha, it was 67% (CI 57-75); against Gamma, 61% (CI 45-72).
Test-negative control study – Skowronski 2021 (preprint)
Canada – Vaccine effectiveness after 2 doses of BNT-Pfizer or Moderna vaccine was close to complete for severe disease (caused mostly by the Alpha variant)
This study was based on everyone in Ontario aged 16 or over who wasn’t living in residential care, and got a Covid-19 test between mid-December and mid-April. Out of the 53,270 people who tested positive, 21,272 had 1 or 2 doses of BNT-Pfizer or Moderna vaccine. Vaccine effectiveness 7 days after the second dose was 91% (CI 89-93), and it was 98% against severe disease (CI 88-100). Effectiveness was lower after the first dose, and it was lower for people aged 70 plus – but after 28 days, their rate caught up to younger peoples’.
They had data on variants available for most of the people who tested positive. The most common strain was Alpha, and some Beta and Gamma. Effectiveness against these variants of concern was not reduced.
Test-negative control study – Chung 2021 (preprint)
UK – People were more likely to get infected after vaccination if they were frail and elderly, or lived in a deprived area; fewer symptoms were reported for breakthrough infections than infections in unvaccinated people
This is an analysis from a study of over a million adults in the UK called the COVID Symptom Study, where people log onto an app. When they report symptoms, they are invited for a test within the program – but the test results need to be reported into the app by the participant. Of the vaccinated people, about 60% were vaccinated with BNT-Pfizer, the others with AZ vaccine.
Between December and the end of April, 2,394 (0.2%) vaccinated people reported that they had tested positive. They matched them one-to-one with people who tested negative, based on date of the test, healthcare worker status, and sex. In a second analysis, they matched them one-to-one on several additional criteria with unvaccinated people who tested positive.
People were more likely to get infected after vaccination if they were frail old people or they lived in deprived areas. Vaccinated people who tested positive reported fewer symptoms than unvaccinated people who tested positive.
Prospective test-negative case-control study – Antonelli 2021 (preprint)
UK – BNT-Pfizer was very effective against hospitalization and symptomatic disease caused by the Delta variant after 2 doses; AZ was very effective against hospitalization, but substantially less against symptomatic disease
This is based on 2 studies from Public Health England. The first was based on the UK-wide Covid-19 testing and sequencing data, linked with vaccination data. The control group was people who tested negative in the same time period. Out of the sequenced test results, there were 11,621 infections caused by Beta detected, and 1,054 caused by Delta. Vaccine effectiveness was around 50% for both BNT-Pfizer and AZ vaccine after a single dose for Beta. After dose 2, however, vaccine effectiveness for BNT-Pfizer was higher at 93.4% (CI 90-96); for AZ, it was 66.1% (CI 54-75).
Numbers for Delta were much smaller, so the uncertainty was higher. Neither vaccine was effective after 1 dose: it was around 33% for both vaccines. After dose 2, however, BNT-Pfizer vaccine effectiveness was 87.9% (CI 78-93); for AZ, it was 59.8% (CI 29-77) – that lower bound of the confidence interval is below the 30 minimum for Covid vaccine efficacy set by the WHO and others.
Vaccine effectiveness against Delta for the 2 vaccines combined in this study after 2 doses was 80.9% (CI 71-88), up to May 16. Data up to June 11 are similar: 79.0% (CI 78-80). And it confirms what’s emerging from laboratory tests (see this summary Twitter thread by David Bauer).
In the second study, with similar methods for data up to June 4, effectiveness against hospitalization with Covid-19 was higher for both vaccines. For BNT-Pfizer, it was 94% after 1 dose (CI 46-99) and 96% after 2 doses (CI 86-99). For AZ, it was 71% after 1 dose (CI 51-83) and 92% after 2 doses (CI 75-97).
Test-negative control studies – Bernal 2021 (preprint) and Stowe 2021 (preprint)
Brazil – Sinovac’s CoronaVac remained effective against symptomatic disease after 2 doses in a Gamma outbreak in people aged 70-74, but not older; no data on protection from severe outcomes
This study of Covid tests in people aged 70 or over in São Paulo covered mid-January to the end of April. By March, the rate of Gamma in the outbreak was over 80%. This study reported results for people getting 2 doses of Sinovac’s CoronaVac, 2-4 weeks apart.
The “cases” in this study were people with Covid-like symptoms getting tested, who hadn’t tested positive recently until this test. They were matched one-to-one with people who tested negative while having Covid-like symptoms in the last 2 weeks of January, were in the same age band (4-year bands), municipality, and race. There were 7,950 matched pairs.
Only symptomatic disease was studied, not severe disease, hospitalization, or death. A single dose of vaccine wasn’t effective. From 14 days after dose 2, vaccine effectiveness was 42% for the whole group:
- Ages 70-74: 61.8% (CI 35-78);
- Ages 75-79: 48.9% (CI 23-66); and
- Ages 80+: 28.0% (CI 1-48).
Test-negative case-control study – Ranzani 2021 (preprint – includes study protocol)
First results in whole-of-town studies
There are 2 whole-of-town studies in Brazil. The first, in Serrana, uses Sinovac’s CoronaVac. The second, in Botucatu, uses AZ vaccine.
There’s background on the Serrana study – Project S – here. It’s a registered stepped wedge trial for all eligible adults in the town, broken into 4 clusters, with volunteers vaccinated with 2 doses of Sinovac’s CoronaVac with a 4-week interval. Vaccination quarter by quarter was spaced a week apart – so there are a few weeks between the first and the last. They finished vaccinating the last quarter of the town on April 11.
There’s no data yet on results between quarters of the town. But the researchers are also comparing what’s happening in the town as a whole compared to a nearby town. There’s not enough data in the first results media announcement, but in the midst of a variant-of-concern driven outbreak around the town, officials report the rate in Serrana dropped. They suggested effectiveness against intensive care was 90% and against death was 95%. And they estimated that the point it which vaccination began to have an impact was when 75% of the people in the trial had been vaccinated. (I think that’s about 60% of the town’s residents – but many people who don’t live there move in and out of the town daily.)
Meanwhile in Botucatu, the second AZ shot is planned for August. The bulk of the first-round of vaccination was done in a single day on May 16, and was accompanied by an increase in testing: 37 days later the Covid hospitalizations had close to halved, and the rate of people testing positive more than halved.
There were 2 other whole-community studies, but one has been canceled. That had been planned in an ethnoreligious community (Hutterite) in Canada, but it was overtaken by the country’s vaccine rollout. The other is in around 46,000 people in Schwaz, in Austria’s Tirol. It became a Beta variant of concern hotspot. People there were vaccinated with the BNT-Pfizer vaccine in March, in a study that will run for 6 months.
My search strategy for these studies of healthcare outcomes: I search PubMed and EuropePMC (because it has a different search engine to PubMed and covers a range of preprint servers) as well as the Cochrane Covid-19 study register, scan the preprints from bioRxiv and medRxiv that are tagged as Covid-19, and do broad searches in multiple languages and countries in Google News daily for reports. I can also come across studies via Twitter.
Study results in people with compromised immune systems
- Although there are differences between diseases, stages of conditions, and treatments, immune responses are often lower in immunocompromised people, especially after a single dose: second doses appear to be critical.
- Immunocompromised people may benefit from a third dose, but there hasn’t been enough study of the impact.
- Some vaccines may have advantages for people with some conditions (for example, Moderna’s), and comparative studies could clarify this – including for mixed vaccine schedules.
- Most of the studies rely heavily on tests of neutralizing antibody response, not the full range of immune response or clinical (Covid-19) outcomes – we need more trials, properly controlled studies, and studies on clinical outcomes.
- Some immune-modifying drugs don’t greatly impair response to mRNA Covid vaccines, but trials are needed on reducing use of those that do temporarily before vaccination. The first report of people doing this with DMARDs (for rheumatic diseases) for a short time didn’t signal a benefit.
- We may start seeing early results from some trials of Covid vaccine in immunocompromised people in July and August, and there is a vaccine specifically designed for people with cancer in early development.
- There is still very little data on non-mRNA vaccines for immunocompromised people.
Vaccination does not work as well for many of the people who are most vulnerable to the virus, including many people with compromised immune systems. It’s a key reason achieving as much community-wide protection against infection as possible is so critical: the less the virus spreads, the lower the risk for those who can’t be vaccinated or for whom vaccination will work less well.
People on immunosuppression therapy and others at high risk of lower immune response were generally excluded from the vaccine efficacy trials. So data on their outcomes after vaccination is only accumulating now in the community, although some clinical trials have also started.
I’ve grouped this section into 3 parts: people with organ transplants or on dialysis; people with cancer; and other people with potentially compromised immune systems. (There’s a good overview here about people living HIV.)
The studies generally consider neutralizing antibodies, but not necessarily the full range of immune response, and too few assess Covid-19 outcomes.
People with organ transplants and people on dialysis
The most studies have been in people on dialysis and with kidney transplants. The team from #NephJC, the Twitter online journal club for nephrology, are maintaining excellent coverage of these, as well as resources generally on Covid vaccination and kidney disease. See their 2 articles:
- An overview of studies and resources curated by Swapnil Hiremath and Joel Topf with a team of contributors and reviewers; and
- An article with charts plotting out the results of studies, by Ed Carr.
They recommend this editorial on Covid vaccination and people on dialysis. Bottom line: people with kidney diseases are developing antibodies, but not as well as healthy people, particularly after the first dose.
Since my last post, there have been several more studies in people in dialysis. There have been so many now, often small and with similar results on antibodies. For people on dialysis, the largest study is still from the US – 1,633 people – with some people who had the J&J vaccine as well as people receiving BNT-Pfizer and Moderna: the overall rate of people with weaker signs of immune response was 22%. People who were sicker had worse results than those who were healthier. From now on, I’m going to focus on studies in dialysis and transplantation that are addressing clinical outcomes – getting the disease, not just immune response – or raise new issues. That’s 2 studies this month:
- A study of 32 people on peritoneal dialysis in Spain who had the Moderna vax found that only 1 person didn’t seroconvert.
- BNT-Pfizer again, Israel: a study of 175 people on dialysis (plus 252 with kidney transplants) found there was immunity till about 3 months, but reduced signs of immune response were associated with more Covid-19, sometimes severe.
Immune responses are much lower for people with transplants, with vaccinated people also more likely to still get Covid-19. There are too many reports now to list here individually. You can see the string of tweets for studies I’ve found since April here (click on “Latest” to sort them by the most recent).
There was some development on the critical question of third doses. In France, for example, 3 doses of mRNA vaccines are already recommended for people who have had organ transplants and other seriously immunocompromised people. A trial of a third dose of Moderna with 120 people in Canada with solid organ transplants has been fully recruited now – they might have early data at the end of July, and another for 80 people with kidney transplants has been registered for Spain too. There is also a trial in Austria of a third vaccine dose of BNT-Pfizer, Moderna, or AZ 4 weeks after 2 doses of either mRNA vaccine in 60 people on the immune-suppressing drug, rituximab.
Two recent reports show why the trial is so necessary: although some people had a more powerful response after the third dose – including some who showed little or no response after 2 doses – results varied. And as serious adverse events are possible for people with transplants, better data is urgently needed. The larger of these came from France, with 101 people with solid organ transplants (BNT-Pfizer), and the other came from the US, with 30 people with solid organ transplants (BNT-Pfizer, Moderna, or J&J). Meanwhile, a report from Belgium included 10 people with kidney transplants who had previously recovered from Covid-19: the authors suggested a single dose of BNT-Pfizer vaccine may have been enough for this group.
Head-to-head trials of the vaccines might be worthwhile too. For example, a small, uncontrolled study in people with lung transplants reported a higher response from Moderna than BNT-Pfizer. Given vaccines came into use at different times, and it’s possible the people vaccinated first were different, we need well-controlled studies to get answers.
People with cancer
This list is all the studies I’ve found so far (including those from the last post). None of these studies is a trial. Many include comparison groups of healthy people, but some don’t. (Most are journal publications, but some are preprints.)
- Blood cancers:
- 2 studies of lymphadenopathy detected by scanning, in 719 people with solid and blood cancers, and in 137 people with blood cancers (after BNT-Pfizer vax) – found it was common and indicated immune response, but the rate was lower in people recently using some treatments.
- A study of BNT-Pfizer vaccine in 95 people with solid cancers and 56 with blood cancers found a low response after 1 dose, and the authors concluded people with cancer should be a priority group for second doses.
- A study of mRNA vaccines in 67 people with blood cancers concluded response was suboptimal. (The rate of positive responses was somewhat higher after Moderna vax, but it was a small number of people.)
- The authors of a study of a single dose of BNT-Pfizer vaccine in 92 people with blood cancers concluded that people with multiple myeloma had very low immune response, especially those on anti-CD38-based treatment.
- Chronic lymphocytic leukemia (CLL): 3 studies, mostly BNT-Pfizer (1 with Moderna) – with 44, 52, and 167 people. Immune response is very impaired, but it varies by disease activity and treatment status.
- Chronic myeloid leukemia (CML): 2 studies, BNT-Pfizer only – 15 and 16 people. The authors concluded immune response might be better than for some other people with cancer.
- Lymphoma: 1 study in 129 people vaccinated with either BNT-Pfizer or AZ. Responses differed among treatments and type of disease.
- Lymphoma and CLL: Another study after BNT-Pfizer or AZ, this time in 53 people, also found low responses that differed among treatments.
- Multiple myeloma (MM): 1 study in 48 older people after 1 dose of BNT-Pfizer vax concluded response rates were low, and the authors concluded second doses were needed. Another study included 28 people with multiple myeloma who had 1 dose of either BNT-Pfizer or AZ vaccine: only 44-50% had an immune response the authors judged as adequate 3 weeks after a single dose. There has also been a report of a woman with multiple myeloma who had no immune response to BNT-Pfizer vaccine dying from Covid-19.
- Myeloproliferative neoplasms: 1 study, 21 people, with a reassuring response to the first BNT-Pfizer vax.
- Solid cancers:
- A study (already mentioned above) of lymphadenopathy detected by scanning, in 719 people with solid and blood cancers after BNT-Pfizer vax, found it was common and indicated immune response– the rate varied.
- A study (already mentioned above) of BNT-Pfizer vaccine in 95 people with solid cancers and 56 with blood cancers found a low response after 1 dose, and the authors concluded people with cancer should be a priority group for second doses.
- The authors of 2 studies in people with solid cancers after BNT-Pfizer vax – 110 and 122 people – also found a weakened response after 1 dose, and strongly recommended second doses as a priority.
- A study reported safety outcomes for 134 people on immune checkpoint inhibitors who had both doses of BNT-Pfizer: other than a higher rate of muscle pain, adverse events were similar.
- Unspecified cancers:
- A study in 89 people with cancer who had Covid-19 found high levels of immune response after a single dose of BNT-Pfizer vaccine, but it’s not known how long it lasts or whether a single dose in cancer patients who recover from Covid-19 provides enough protection.
And in other news, preclinical (animal study) results have been posted for a Covid vaccine in development in California that’s specifically for people with cancer. It’s a viral vector vaccine based on oncolytic herpes simplex virus-1, and it aims to enhance anti-tumor response as well as protecting against Covid-19.
Other people with potentially compromised immune systems
This list is all the studies I’ve found so far (including those from the last post). None of these studies is a trial. Many include comparison groups of healthy people, but some don’t. (Most are journal publications, but some are preprints.) It’s going to be a while till a very clear picture emerges about some immune-modifying drugs.
- Rheumatoid arthritis and psoriasis:
- A prospective controlled study of 910 adults with rheumatic diseases and 182 age- and sex-matched healthy people were vaccinated with 2 doses of CoronaVac with a 4-week interval in São Paulo, Brazil. Adverse reactions weren’t different, but signs of immune response were lower in the people with rheumatic disease. The participants were all vaccinated within 2 days, and data on Covid-19 in the groups will be collected for a year. Soon after they were vaccinated, an outbreak of Gamma broke out in São Paulo. The authors noted that the rate of infection in the vaccinated group decreased rather than increasing with the general community outbreak.
- A study of 686 people with autoimmune inflammatory rheumatic diseases after BNT-Pfizer vaccine in Israel (with 121 people from the general population for comparison) – most people developed a good immune response, but it was impaired by some treatments: severely by rituximab; moderately by glucocorticoids, abatacept, and mycophenolate mofetil; and only mildly by methotrexate.
- The first study I’ve seen reporting on outcomes when people were advised to temporarily stop some disease-modifying drugs (DMARDs) for rheumatic diseases has been published. It wasn’t a trial, though the authors recommend trials be done. The people who took the break didn’t have a better immune response than those who didn’t follow the advice. In this group of 140 people from Italy, anticytokine drugs didn’t seem to affect response from BNT-Pfizer vaccine, but methotrexate and glucocorticoids did.
- A study in 264 people with inflammatory rheumatic diseases after BNT-Pfizer vaccine. Small numbers for people on some treatments; low response for people on B cell-depleting agents; somewhat reduced for people taking methotrexate, but generally when in combination with other drugs.
- A study in 83 people on immune-modifying drugs for psoriasis, psoriatic arthritis, and rheumatoid arthritis, found responses to BNT-Pfizer vaccine were high after biologics, but lower after methotrexate.
- A study in 53 people with rheumatoid arthritis on disease-modifying drugs (DMARDs) after mRNA vax (9 people had Moderna, the rest had BNT-Pfizer). The authors concluded their response was different to healthy people’s, and protection may rely on the second dose.
- A study in 136 people with autoimmune rheumatic diseases who got AZ (Covishield) or Covaxin (Indian inactivated vaccine): on average, response was lower for Covaxin than AZ, and both were lower than healthy people’s responses.
- A study in 910 people with autoimmune rheumatic diseases (ARD) (most on immunosuppressive drugs) vaccinated with Sinovac’s CoronaVac, matched to 182 healthy people – during Brazil’s outbreak of Alpha and Gamma variants of concern. The authors concluded people’s immune responses were reduced but acceptable. During the study, 4% of the ARD group were diagnosed with Covid-19 (36 people) versus 1.6% in the control group, mostly with Gamma. Of the 36 people with ARD with Covid-19, 4 were hospitalized – each less than 10 days after the second dose – and none died.
- A report of 3 people taking apremilast for psoriasis: they did not experience disease flares after Covid vaccination (1 had AZ vaccine, and the other 2 had BNT-Pfizer).
- Multiple sclerosis (MS):
- A study in 125 people with MS, a month after a second dose of BNT-Pfizer vaccine. People taking no disease-modifying drug or cladribine had a strong response to vaccination; it was low in those taking ocrelizumab; and vaccination didn’t seem to work in people taking fingolimod. In a letter in response to this, some authors wrote that there isn’t enough evidence to justify the risk of changing disease-modifying treatment before vaccination.
- A report of 4 people with MS in Italy, suggesting ocrelizumab might reduce immune response after BNT-Pfizer vaccine.
- Non-Alcoholic Fatty Liver Disease (NAFLD):
- A study in 381 people with NAFLD after Sinopharm’s Beijing vaccine found a strong response to the vaccine and a low rate of adverse reactions.
- Inborn errors of immunity:
- A study in 26 people with inborn errors of immunity, most on immunoglobulin therapy, after BNT-Pfizer vaccine: most, but not all, developed immune responses.
- Mixture of reasons for immunocompromise:
- A study of 82 people with immune-mediated diseases after 2 doses of Sinovac’s CoronaVac. They had a lower rate of antibodies than healthy controls (but higher than a control group of people 65 years and older), but it was still high. However, people on immunosuppressive or immune-modifying medications had much lower signs of immune response. The authors concluded a third dose for this group should be studied.
I tweet about these studies and recently began tagging them: you can keep up with those on Twitter via this search – and then click on “Latest”, to see them in reverse chronological order so you can see the ones I find after this post. A caveat: my searches for these studies are less extensive than my searches for clinical trials.
The landscape after new data: 11 vaccines with enough phase 3 results
Critical caveats on the data presentation that follows:
With a single exception (Sinopharm), each vaccine has only been tested alone, and we don’t have a rigorous basis for comparing them. On the other hand, with 11 vaccines around the world with enough phase 3 results, it’s hard to keep track of them and keep them in perspective. (The 4 that don’t have enough for this are Abdala, CureVac, Soberana, and ZyCov-D.) I decided an oversimplified “sketch” of this landscape was more likely to help with that perspective, than mislead. But keep in mind:
- The number of people in the trials, and the quality of those trials (and the amount of data available), all vary greatly. That means that there’s a great deal more certainty for some vaccines than others.
- Vaccine efficacy is a relative reduction in the size of risk of disease – not an absolute drop in percentage points of a risk that is 100%.
- The participants can be very different. For the vaccines which have much higher rates of adverse events in people under 55 than over, the age spread in trials could affect that considerably.
- Some of the vaccines have been tested against variants of concern that reduced their efficacy, while others have not.
- Some of the vaccines reach their peak efficacy sooner than others, and their efficacy rates are likely to improve when later results come in.
- Efficacy can be different according to the interval between doses, and that interval isn’t the same in every trial.
The data behind 2 vaccines is very preliminary – just a few data points in a press release (CanSino and Covaxin). So vaccines will shift positions, sometimes even a lot: they’re like counters that could move around on a board.
The top left is where vaccines with high efficacy and low rates of adverse events would sit – and the bottom right is lower efficacy and higher adverse events. They all “work” though. The evidence I used to manually position the counters is in the table below. They were numbered in the order in which efficacy results for them were made public.
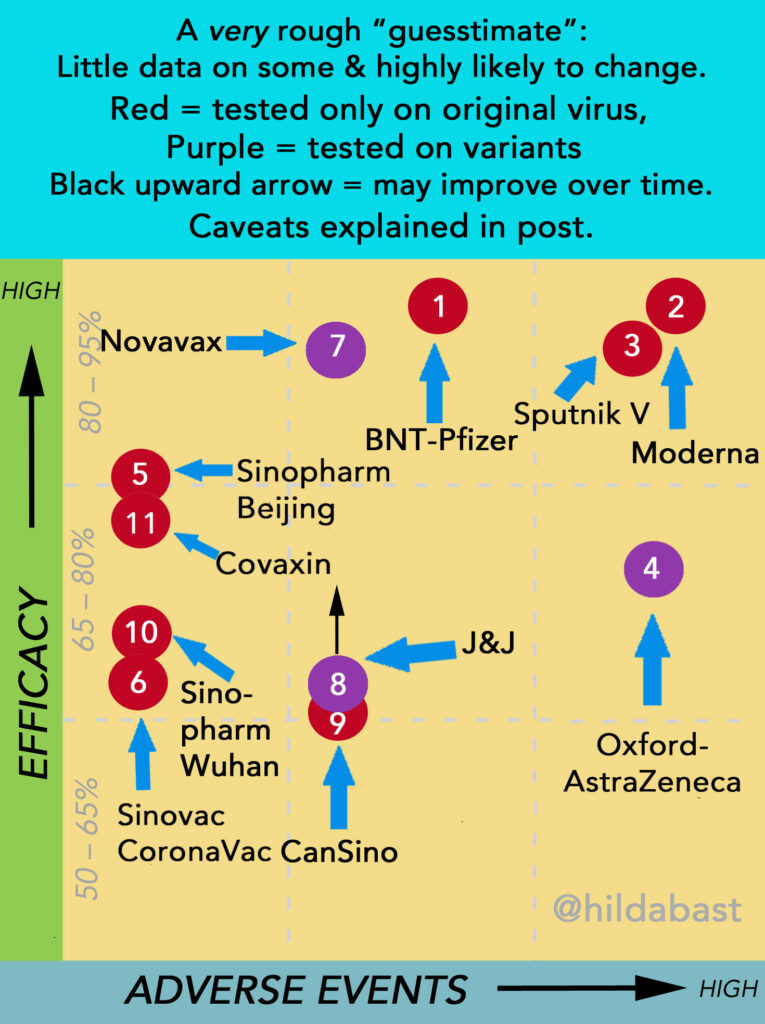
| Key | Vaccine | Efficacy (CI*) | Data |
| 1 | BNT-Pfizer | 95% (90-98) At 6 mths: 91% (89-93) | >43k people (half on placebo) Reviewed & detailed reports public; protocol public Evidence: strong |
| Most common systemic adverse event: fatigue (56%) Most commonly severe: fatigue (4%) | |||
| 2 | Moderna | 94% (89-97) At 6 mths: 90% (?) | >30k people (half on placebo) Reviewed & detailed reports public; protocol public Evidence: strong |
| Most common systemic adverse event: fatigue (65%) Most commonly severe: fatigue (10%) | |||
| 3 | Sputnik V (Gamaleya) | 92% (86-95) | >22k people (3:1 placebo) Reviewed & detailed report public; protocol not public Evidence: moderate-strong (reservations about methodology); adverse events not routinely solicited in a representative group in phase 3, data from San Marino study |
| Most common systemic adverse event: asthenia (fatigue) (32%) Most commonly severe: asthenia (around 5%) | |||
| 4 | Oxford/AstraZeneca | 76% (68-82) (US/LatAm trial) Other trials: 63% (52-72) 76% (59-86) (delayed 2nd dose) (Standard dose, February data, includes “UK”, “SA” strains) | >17k people in grouped trials (half in control group, half on varied vaccine regimens), >32k in US/LatinAmerican trial (two in vaccine to each in placebo) Reviewed & detailed reports public; press release only for US/LatAm trial; protocols public Evidence: strong from US/LatinAmerican trial, though weak to moderate from group of trials with reservations about methodology; adverse event data from MHRA, rare risk from EMA |
| Most common systemic adverse event: fatigue (62%) Most commonly severe: malaise (4%) Thrombosis & Thrombocytopenia Syndrome (TTS): 1 in 100,000 | |||
| 5 | Sinopharm – Beijing | 81% (67-89) (up to age 60) | 15k people (same on placebo) Reviewed by WHO; detailed report and protocol public Evidence: strong |
| Most common systemic adverse event: headache (13%) 0.1% severe | |||
| 6 | CoronaVac (Sinovac) | 50% (35-62) (Anvisa, Brazil) 84% (65-92) (Turkey) | Brazil >12k people (half on placebo) Reviewed by Anvisa & WHO, trial and protocol public Turkey >10k people (half on placebo) Reviewed by WHO; protocol not public Evidence: moderate (further trials in progress) |
| Most common systemic adverse event: fatigue (<2%) No severe event reached 1% | |||
| 7 | Novavax | 90.4% (83-95%) (US/Mexico trial, with variants) 96% (Original strain only, UK trial) (74-99.5) | 30k people (a third on placebo) (US/Mexico trial) Some details and protocol public Evidence: strong >14k people (half on placebo) (UK trial) Detailed report and protocol public Evidence: moderate Note: the results from a small phase 2b trial in SA showed it worked, but with much lower efficacy because of the “SA” strain (perhaps 49% or 60%). However the evidence was very weak. |
| Most common systemic adverse events: fatigue/headache/muscle ache: around 40% Most commonly severe: fatigue (around 3%) | |||
| 8 | J&J | 66% at 28 days (Including “SA” strain) (55-75) ~70% after 56 days; 72% at 28 days USA (58-82) | >43k people (half on placebo) Reviewed, detailed report; protocol public Evidence: strong (further large trial on 2 shots in progress); rare risk from CDC & EMA |
| Most common systemic adverse event: fatigue (38%) Most commonly severe: fatigue (1%) Thrombosis & Thrombocytopenia Syndrome (TTS): about 1 in 400,000 | |||
| 9 | CanSino | 69% at 14 days (?) 65% at 28 days (?) | ca 40k people (half on placebo) Press release only; protocol not public Adverse event data only available for small early phase trial Evidence: potentially strong (too little known) |
| Most common systemic adverse event: fatigue (34%) None severe reached 1% | |||
| 10 | Sinopharm – Wuhan | 73% (58-82) 2nd trial stopped for lack of efficacy | 15k people (placebo group same size) Detailed report and protocol public 2nd trial 6k people (half on placebo) No data released Evidence: strong |
| Most common systemic adverse event: headache (13%) 0.1% severe | |||
| 11 | Covaxin | 78% (65-86) | Under 26k people (half on placebo) Detailed report public; protocol not public Adverse event data from earlier phase trial Evidence: potentially strong (too little known) |
| Most common systemic adverse event: headache (1%) Severe not reported (none serious) |
What else is new for the 14 vaccines with efficacy results
Abdala
The Abdala vaccine is a protein subunit vaccine with a 3-dose course, at 2-week intervals – so people are fully vaccinated in a month. It was developed by the public Centro de Ingeniería Genética y Biotecnología (CIGB) (Centre for Genetic Engineering and Biotechnology). When Covid-19 began to surge in Cuba in May, the vaccine was rolled out before phase 3 results were in.
The Abdala trial was, I think, the fastest to recruit of all the large Covid vaccine trials. It began recruiting in late March, and recruited 48,000 in about a month. On June 22, CIGB announced the vaccine had 92.3% efficacy. There is no indication of how much uncertainty there is around this result: since it came so quickly, there might not have been a lot of “events” (people with Covid-19), but we won’t know till further data is released. The protocol for this trial hasn’t been released, so we don’t know how high the bar was set – although they have said they meet the WHO criteria for success, which is a lower bound of at least 30% for the confidence interval around efficacy.
I haven’t included this vaccine in the landscape infographic yet, because there is nothing to give an indication of the rate of adverse events, with no data on this in the efficacy announcement, and no data published from early phase trials.
Venezuela will be running a trial with this vaccine, and plans to produce it locally.
The Abdala vaccine is named after a poem by José Martí (1853-1895) about a fictional country struggling for liberation.
Records on this vaccine in my collection here.
The moment the team behind Abdala heard the efficacy result:
Janssen-Johnson & Johnson (J&J)
The J&J vaccine is a single-dose viral vector vaccine, based on Adenovirus 26. Since my last post, the first reports for Sisonke were released – that’s the implementation study in South African healthcare workers. It’s run by the SA Medical Research Council. They published a research letter in NEJM about thromboembolic events in Sisonke: in the first 288,368 people, there were no cases of TTS (thrombosis and thrombocytopenia syndrome). (South African Olympic teams are also being vaccinated through Sisonke.) They have also notified that most breakthrough infections are mild, with 4% moderate and 2% severe.
In other news:
- J&J sold 200 million doses to the COVAX system, to be delivered in 2021, with an option for another 300 million in 2022. (COVAX now expects to distribute 1.9 billion doses in 2021 – all for 2-dose vaccination, except for J&J.)
- Production problems in the US and the safety pause there happened at the same time as the US vaccination rate dropped (as was predicted), leading to low use of the vaccine there.
- Janssen/J&J started a trial of the same amount of active vax in 0.3ml instead of 0.5ml: if those are equivalent, then the same bottling capacity – one of the bottlenecks in vaccine supply globally – could be sending out more vaccine doses.
This vaccine is listed by WHO and distributed in the COVAX scheme.
Records in my collection for this vaccine here. Includes 4 laboratory studies on variants, plus clinical data in the international phase 3 trial.
This vaccine also in the sections on mixed vaccine schedules and immunocompromise.
CanSino
CanSino is a single-shot viral vector vaccine, based on Adenovirus 5 (trade name, Convidecia): we still don’t know if this vaccine causes TTS as 2 other adenovirus-based vaccines do.
This vaccine ended its partnership with a Brazilian company and its application for authorization in Brazil has been closed.
This vaccine is being evaluated for WHO listing. Records in my collection for this vaccine are here – no studies on variants. A summary of the minimal phase 3 data released so far for this vaccine is in my March post.
This vaccine also in the section on mixed vaccine schedules.
BNT-Pfizer
Some bad news this month: along with the Moderna vax, the CDC concluded that there is a rare risk of heart inflammation (myocarditis or pericarditis), usually after the second shot, and usually in adolescents and young men. It generally resolved with treatment and rest, and has been milder than usually expected for this condition. As of June 28, the CDC and FDA confirmed 518 cases in people under 30: there had been over 8 million doses of these vaccines in males in that age group up to June 11 (very roughly 1 in 15,000).
In other news for this vaccine:
- Pfizer has begun a trial of co-administration of the BNT-Pfizer vaccine and pneumococcal vaccine (a vaccine against bacteria that cause pneumonia, blood infections, and meningitis).
- A study of the vaccine and memory B cells supports the idea that the vaccine could provide protection against variants over time.
- The US ordered half a billion doses of this vax for COVAX – 200 million of those doses to be distributed this year.
- Norway released results of its study of very frail older people who died after BNT-Pfizer vax: they described them as having been already “in the process of dying” and unable to cope with the vaccination.
This vaccine is listed by WHO and distributed through COVAX.
Records in my collection for this vaccine here. Includes 47 laboratory studies on variants as well as clinical trial data.
This vaccine also in sections on mixed vaccine schedules, immunocompromise, and community impact studies.
Oxford-AstraZeneca
AstraZeneca has started a phase 2/3 trial of a version of the vaccine adapted for the Beta variant of concern – but it didn’t seem to be registered when they announced this. It’s for 2,250 participants, in the UK, South Africa, Brazil, and Poland. (There’s a preclinical study on this formulation.)
In June, the developers reported on a booster study. People who got 1 or 2 shots in early trials were invited back for a boost: 30 got a second shot after many months, and 90 got a booster after having had both shots. Signs of immune response increased. The authors concluded that a lengthy delay improved response. (Note, it wasn’t a randomized comparison of length of dosing interval.)
In other news, the European Medicines Agency (EMA) added another very rare side effect to the product information for this vaccine, to warn people who have had the rare condition, capillary leak syndrome, that the vaccine could trigger another episode.
This vaccine is listed by WHO and distributed through COVAX.
Records in my collection for this vaccine here. Includes 10 laboratory studies on variants, as well as clinical trial data on Alpha and Beta.
This vaccine also in sections on mixed vaccine schedules, immunocompromise, and community impact studies – including a whole-of-town study update.
CureVac
This vaccine’s interim results are “the big miss” in this post’s title. The vaccine is a nanoparticle mRNA vaccine that doesn’t require super-freezing. The first sign it might not be very powerful came in some preclinical results in December. Then the big phase 3 trial had its first interim analysis, but didn’t release the results. On June 16, the company announced its second interim results: overall efficacy was 47%, and they said it reached efficacy for people under 60, but not over.
On June 30, they announced final results, based on the 228 events (symptomatic Covid-19). Overall efficacy was 48%, and in people aged 18-60, it was 53%, with 77% against moderate and severe disease (9 in the vaxed group, 36 in placebo). There were no hospitalizations of deaths in the vaxed group, versus 6 in placebo.
There wasn’t detail on adverse reactions in their press release, but in their early phase results, the rate of adverse events was high in the 12µg dose that went to phase 3 trial, though the number of people was very small – 27 of 28 people reported fatigue (compared to only 17% in the placebo group).
CureVac is planning to take a second version of the vaccine, CV2CoV, into clinical trial this year. They’ve published a preclinical study on that new vaccine.
Why were the results so weak in comparison to the first 2 mRNA vaccines? The company pointed to variants as a big issue, but only around half of the infections they sequenced were caused by variants of concern. Others are speculating that it might be because of their use of unmodified mRNA, but there could be other reasons.
The company is continuing to develop its planned RNA vaccine printer.
Records on this vaccine in my collection here.
Moderna
Spikevax! That’s the commercial name they’ve nabbed for this vaccine in Europe. That’s a relief – after Comirnaty and Vaxzevria, I was starting to wonder if there was a competition for hardest name going on!
Some bad news this month: along with the BNT-Pfizer vax, the CDC concluded that there is a rare risk of heart inflammation (myocarditis or pericarditis), usually after the second shot, and usually in adolescents and young men. It generally resolved with treatment and rest, and has been milder than usually expected for this condition. As of June 28, the CDC and FDA confirmed 518 cases in people under 30: there had been over 8 million doses of these vaccines in males in that age group up to June 11 (very roughly 1 in 15,000).
In other news, Moderna registered a trial for a multivalent Covid vaccine, combining their original formulation with the version adapted for the Beta variant of concern.
This vaccine is listed by WHO and is being distributed through COVAX.
Records in my collection for this vaccine here. Includes 10 laboratory studies on variants, including a preclinical study of the adapted vaccine adapted for a variant.
This vaccine also in sections on mixed vaccine schedules, immunocompromise, and community impact studies.
On June 26, the WHO reported that the COVAX cupboard was empty – and then a trickle of Moderna got through:
Novavax
This is the “big hit” in this post’s title. It was a trial readout of the US/Mexico trial, with 29,960 participants (2:1 randomization with placebo), confirming the high efficacy results of the UK trial (96.4% efficacy against the original strain, 86% against Alpha):
- Overall vaccine efficacy against symptomatic Covid-19: 90.4% (CI 83-95);
- The only moderate and severe cases of Covid-19 were in people getting placebo (14 people);
- Out of the 77 cases of Covid-19, they had sequenced 54 so far – and efficacy against variants of concern or interest was 93.2% (CI 84-97), mostly Alpha. (Note: it had reduced efficacy against Beta in its South African phase 2 trial, though the numbers were too small to be sure by how much.);
- High diversity in the trial – 20% Latin American, 12% African American, 7% Native American, and 5% Asian American;
- The most common systemic adverse events were fatigue, muscle pain, and headache, each at around 40% – for the 2 mRNA vaxes, that’s over 50% and 60%. (I wrote about the implications of this in The Atlantic.)
The other major news for this vaccine were the results of small subgroup in their UK trial, where people got a flu vaccine and Novavax at the same time: 217 got a flu vax and 214 a placebo second injection. The flu vaccines were by Seqirus, Flucelvax and Fluad. Co-administration of the vaccines didn’t seem to reduce the immune response to Novavax, but it did add about 10% to the adverse reactions.
In other news for this vaccine:
- Novavax released preclinical results for a version of the vax adapted for Beta.
- The results of the UK trial, which had been released in a preprint, were published in the NEJM.
- The Serum Institute of India (SII) began commercial scale manufacturing of this vaccine. It will be called Covovax there. The trial testing its equivalence to the Novavax version is still going on: SII anticipates a September launch.
- The company anticipated producing 100 million doses a month by the end of September, and 150 million doses a month by the end of 2021.
It’s not clinical data holding up its authorization, so it’s presumably something to do with manufacture, which also has to satisfy drug regulators’ requirements. It’s expected to be authorized first in the UK and South Korea, where there is local manufacturing, then Europe, and then WHO. No word on when it’s expected in the US.
This vaccine is being evaluated by WHO.
Records in my collection for this vaccine here. Includes 2 laboratory studies on variants and clinical trial data.
This vaccine also in the section on mixed vaccine schedules.
Soberana
The Soberana with results is a conjugate vaccine – it combines protein subunit with tetanus toxoid. This type of vaccine has been used since the 1980s – for example, Hib vaccine. The vaccine is produced by the public Finlay Institute in Cuba – like Abdala, this vaccine is government-owned, not-for-profit, and when Covid-19 began to surge in Cuba in May, the vaccine was rolled out before phase 3 results were in, too. There is a technology transfer agreement for local production in Ghana, and it will be produced in Iran too, where 24,000 participants have been recruited in a phase 3 trial.
There are 3 versions of this vaccine: Soberana 01, a non-conjugate vaccine; Soberana 02, which is the version in the phase 3 trial; and Soberana Plus, which is a booster. A phase 3 trial for Soberana 01 is planned, comparing it with Soberana 02, not placebo.
Efficacy for the first 2 doses in the Cuban trial for Soberana 02 have been announced: 62%. As with the Abdala vaccine, there is no indication of how much uncertainty there is around this result: since it came so quickly, there might not have been a lot of “events” (people with Covid-19), but we won’t know till further data is released. The protocol for this trial hasn’t been released, so we don’t know how high the bar was set – although they have said they meet the WHO criteria for success, which is a lower bound of at least 30% for the confidence interval around efficacy. There have been no published studies on Soberana 02, but there is a preprint for phase 1 results for Soberana 01.
This month, a phase 1/2 trial for 3- to 18-year-olds began, with Soberana 02 and Soberana Plus. No control group is planned, and it’s for 350 participants, with safety and signs of immunity outcomes.
I haven’t included this vaccine in the landscape infographic yet, because there is nothing to give an indication of the rate of adverse events, with no data on this in the efficacy announcement, and no data published from early phase trials for Soberana 02.
Records on this vaccine in my collection here.
Sputnik V
In my last roundup, I dug deeply into the wide range of conflicts and issues around the scientific testing, manufacturing quality, and disinformation campaign around this vaccine. Given the problems and uncertainties I discussed related to the quality of the clinical trial program, rigorous studies from someone other than the manufacturers of this vaccine are critical to get a reliable perspective on this vaccine’s efficacy. I still haven’t been able to identify a solid community impact study for this vaccine. There is a trial going on in the UAE, so that’s an important one to look out for, even though it only has 1,000 participants. And as we’ll see below, there may be strong studies coming from Brazil. In Argentina, researchers have reported on immune responses after 1 dose in people who were previously infected with SARS-CoV-2.
The developers now claim that the vaccine has been shown to be almost 92% effective against variants of concern, but that’s based on unpublished “digital medical and vaccine records”. However, laboratory studies from the US and Argentina found reduced immune responses to some variants of concern. The authors of the Argentinian study stressed the need for the second dose. Also, from Argentina, researchers have said they found encouraging results for the Lambda variant of interest, and perhaps less worrisome findings for variants of concern – that study hasn’t published. (Variants of interest are less of a worry than variants of concern.)
The WHO’s evaluation of the vaccine currently has this status: still not enough data provided for preclinical studies, clinical studies, and chemistry, manufacturing, and quality control processes. In other words – not enough data on anything. They are also following up on the issues their factory inspections identified. WHO inspectors reported grave issues at 1 of the 4 factories, including concerns about the integrity of data and test results, traceability of what’s produced, adequacy of sterile procedures, and risk of cross-contamination. Russia responded on June 23 that they had already addressed the concerns, but WHO hasn’t issued an all-clear, as far as I know.
The developers report that they have been using the vaccine in a nasal spray in children aged 8-12, and said there will be trials of this. They also say they expect the vaccine to be authorized for use in teens by September – apparently without clinical trials in this age group.
Meanwhile, there were developments in the 2 countries reporting battles between regulators and the Sputnik developers I discussed last time. Slovakian politicians over-ruled the nation’s drug regulators, with the cabinet authorizing the use of the batch the country that had bought. In announcing the decision, the Health Minister said he wouldn’t use it or recommend it. Since then, they’ve announced plans to sell it or donate it, because too few people are willing to take it.
In Brazil, Anvisa, the national drug regulator, threaded the needle over the political pressure to authorize the vaccine despite the agency’s concerns after a 7-hour meeting with a 4 to 1 vote: they allowed the states that wanted to import the vaccine to do so, but under strict conditions. The vaccine must only come from the factories they were allowed to inspect, each batch must be tested locally, and it must only be used in an effectiveness study.
Supply problems continue, especially for the second dose (which is a different vaccine to the first dose). Argentina is reportedly about to start producing that second vaccine (based on Adenovirus 5). Other countries are also gearing up to produce the vaccine, including Iran. Russia itself is beginning to impose sanctions on those who don’t vaccinate, in the face of a coronavirus surge and very low vaccine uptake domestically (12% fully vaccinated). And although they have said (without releasing data) that the vaccine is effective for longer than 6 months, Moscow has begun a booster campaign for everyone vaccinated 6 months ago. Vaccination will reportedly be twice a year until at least 60% of the community is vaccinated.
This vaccine is being evaluated for WHO listing. Records in my collection for this vaccine here, including 2 laboratory studies variants of concern.
This vaccine in also in the section on mixed vaccine schedules.
Sinopharm – Beijing
The phase 3 trial was published in JAMA. After such a long wait for data from this trial, it didn’t seem like a coincidence that we saw it just after WHO had released data on this vaccine.
In other news for this vaccine:
- An immunobridging trial in 3-17 year-olds in the UAE was registered. (The other data in under-18s is in China, with limited ethnicity.)
- The UAE began offering a third shot of the vaccine 6 months after the second dose, with a priority for older people and those with chronic disease.
This vaccine is listed by WHO and is eligible for distribution through COVAX.
Records in my collection for this vaccine here. Includes 2 laboratory studies on variants.
This vaccine also in section on immunocompromise.
Sinopharm – Wuhan
Phase 3 trial data were published at the end of May. This was the #4Humanity trial in the UAE and several other countries in the region, but not all the countries’ data is included in this publication. There were 3 groups in the trial – this vaccine (WIV04), Sinopharm’s Beijing vaccine, and a shared control group given the adjuvant alone. There were very few women in this trial – only 16% in the Wuhan vaccine group.
The trial concluded the Wuhan vaccine had an efficacy rate 14 days after the second dose of 72.8% (CI 58-82). No one had severe Covid-19 in either vax arm – 2 people got severe Covid in the control group. Efficacy against both symptomatic and asymptomatic Covid-19 was 64.0% (CI 49-75). The most common systemic adverse event was headache (13%): severe headaches were rare (0.1%). (More on this terminology in my explainer.)
This vaccine has also had a trial stopped for lack of efficacy in Peru (discussed in a previous past).
This vaccine is being evaluated for WHO listing.
Records in my collection for this vaccine here. There are no studies on variants on the Wuhan vaccine.
Sinovac CoronaVac
Some major news for this vaccine this month: the WHO listed the vaccine, for use with a 2-4 week interval. The first signs that the whole-of-town vaccination trial in Serrana, Brazil, may have provided a lot of protection the from hospitalization, intensive care, and death from Covid-19. (More above in whole-of-town studies.) And the results of its phase 1 and 2 trials for children and young people – with 550 participants – were published.
The trial compared the same dose as for adults (3μg) and a half-dose, and there was a control group of adjuvant only for the phase 2 trial. There were 2 doses, 4 weeks apart. All the participants were of Han ethnicity – the trials were done in China. Based on these results, Sinovac decided on going ahead with the full-dose, and it has been authorized for use in children in China. The vaccine – at both doses – induced stronger immune responses than they had recorded for adults.
The rate of adverse reactions was very low – the highest systemic reactions were at 4-5% (fever and cough), and only 1 child had a severe systemic reaction (fever). There was also only 1 serious adverse event, and it was considered unrelated to the vaccine. (That case was pneumonia, in a participant in the adjuvant-only group.) (You can read about the terminology for adverse events in my explainer post.)
In other news:
- CoronaVac is the first established vaccine to go head-to-head with another vaccine in a major phase 3 trial without a placebo group: it’s the control group’s vax in TurkoVac’s 40,800-participant trial.
- The phase 1 trial of a third shot of CoronaVac has results, although little has been released: reportedly had a major impact on antibody levels.
- In Chile’s latest national data release they estimated an effectiveness rate of 63.6% against symptomatic disease (CI 63-64); 87.3% against hospitalization (CI 87-88); 90.0% against intensive care (CI 89-91); and 86.4% against death (CI 85-87). This is not a controlled study, but they do adjust for age, gender, region, income, and nationality. (Chile has a 4-week interval for CoronaVac, as the Serrana trial did, whereas the interval was only 2 weeks between doses in the Brazilian phase 3 trial: that might have made a difference.)
This vaccine is listed by WHO. Records in my collection for this vaccine here. Includes 3 laboratory studies on variants of concern, and there is some clinical data on Gamma in community studies.
This vaccine also in sections on mixed vaccine schedules, immunocompromise, and community impact data (including whole-of-town).
Covaxin
This vaccine now has full approval in India, and its developer, Bharat Biotech, has started the process for WHO evaluation for COVAX. The US FDA has informed Bharat’s US partner, Ocugen, that as with all new vaccines that aren’t well-advanced in their process, they will need to apply for full approval, not emergency use authorization there. Ocugen is still applying for emergency use authorization in Canada. The vaccine has been authorized in Brazil, with particular conditions, such as the provision of more safety data from the phase 3 trial – but the procurement contract through a third party has been suspended after a corruption scandal.
[Update July 2] A preprint of phase 3 trial results was posted:
A phase 2/3 trial in 2- to 17-year-olds has started, for 525 participants, with outcomes for safety and signs of immunity. And a phase 3 trial for 4,500 adults was given the green light in Brazil.
The adjuvant for this vaccine was developed in the US with funding from the NIAID (NIH), and they put out a press release about it. It’s called Alhydroxiquim-II and it attaches a small molecule to Alhydrogel (the common vaccine adjuvant called alum). This adjuvant goes straight to nearby lymph nodes without circulating in the body.
Records for this vaccine in my collection here. Includes 4 laboratory studies on variants of concern.
This vaccine is also in section on immunocompromise.
ZyCov-D
This is the first DNA vaccine for Covid, although the vaccine type is not new. Instead of using part of the virus to create the vaccine, it uses a small part of the virus’ genetic code – the DNA plasmid – to trigger the body’s immune system.
The developers, Zydus Cadila, reportedly revealed to investors in a phone call that an interim analysis of their 28,000-participant trial in India had a vaccine efficacy rate of 66.6%. That trial included 1,000 participants aged 12-18 years. It was a 3-dose course. No one in the vaccinated group developed severe Covid-19 in this analysis. And they are applying for emergency use authorization in India.
They also reported that tests showed that a 2-dose course resulted in similar immune responses.
As with Abdala and Soberana, I can’t include this vaccine in the infographic because there hasn’t been enough data released from clinical trials.
Records in my collection for this vaccine here.
Overview of phase 3 trials and what’s new
- There are now 30 vaccines in, or about to be in, phase 3 trials – including 3 additions since the last post, the first protein subunit vaccine from Vietnam (Nanocovax) and one from Sanofi/GSK (CoV2 preS dTM), as well as an inactivated vaccine from Turkey (TurkoVac). (One vaccine was removed from the list.)
- Another 6 vaccines at least are approaching phase 3, all based on mRNA, protein subunit, viral vector, or DNA.
- And another will be discontinued without reaching phase 3: Altimmune’s nasal spray.
The 3 additional vaccines in phase 3 trials underway since my last round-up are:
- CoV2 preS dTM: Sanofi and GSK’s adjuvanted protein subunit vaccine started recruiting in the US, with Mexico signed up and other countries expected participating as well. It’s a trial for 37,430 people.
- Nanocovax: the first protein subunit vaccine from Vietnam by Nanogen has gone with the lowest dose tested in the early research phase. It’s a trial for 13,000 people, with other countries to be announced.
- TurkoVax: Formerly called Erucov-Vac, this is an inactivated vaccine from Turkey.
These vaccines are planning to go into phase 3 soon are:
- Medigen‘s protein subunit vaccine, MVC-COV1902, is going into phase 3 trial in Paraguay. (It’s from Taiwan, and they’re already applying for emergency use authorization based on a large phase 2 trial – 3,700 people – and bridging data comparing it to AZ vax.)
- Another mRNA vaccine that doesn’t need super-freezing: from Arcturus, a US company working with a university in Singapore.
- Israel’s BriLife (IIBR-100) may be close to starting a phase 3 trial in Argentina, as a start. It’s a viral vector vaccine (based on vesicular stomatitis virus, VSV).
- A protein subunit vaccine from Westvac in China, that has 3 doses at 3-week intervals, has been registered, with a planned start in June (no word yet that it has started).
- Inovio’s DNA vaccine, INO-4800, recently lost the US funding for its phase 3 trial. They’re now planning their trial with private funding for vaccine-underserved countries in Asia and Latin America – and they’re concentrating on Asia for the vaccine’s future.
- A phase 3 trial for another DNA vaccine, from South Korea’s Genexine, is on the horizon.
One vaccine has been deleted from this overview for now: UB-612 didn’t go ahead with its phase 3 trial in Brazil because a variant of concern was dominant. However, now they are planning to go ahead in India – if avoiding variants is the goal, it’s not panning out. And Altimmune’s single-dose nasal spray is being discontinued completely after poor results in phase 1.
The table below is updated, so check out any vaccine you’re interested in.
Addendum: The phase 3 trial tables
Phase 3 trials: recruitment progress
| Vaccine | Target or final size | Single or similar trials? | Reaching target? |
|---|---|---|---|
| Abdala CIBG/ BioCubaFarma Cuba |
48,000 | 1 large trial; further small trial planned | Fully recruited – efficacy briefly reported. |
| Ad26.COV2-S Johnson & Johnson USA |
73,783 | 2 large trials | Single-dose trial with 43,783 reported. Second 2-dose trial advanced, perhaps nearing recruitment. |
| Ad5-nCoV CanSino China |
40,500 | Mostly single trial | Press release readout for results of single-dose trial with ca 40,000. |
| ArcoV Walvax/Chinese Academy of Military Science |
28,000 | Single trial | Had planned to start in May. |
| BECOV2 (Corbevax) Biological E India |
Not yet known | Single trial | Approval announced by company on April 24. |
| BNT162b2 BioNTech/Pfizer Germany |
51,945 | Mostly single trial | Trial with 46,331 aged 16 and over reported; results for additional 2,260 people aged 12 to 15 reported in press release. Trial for 4,000 pregnant women began in February. |
| ChAdOx1 nCov-19/AZD1222 AstraZeneca UK (Oxford Uni) |
54,690 | 3 very different large trials (plus some small) | 2 major trials 10,000+ each (UK and Brazil) reported. Major trial in US and other countries, press release report for 32,449. Small trial in India has final results, but not published. |
| CoV2 preS dTM Sanofi/GSK France |
37,430 | 1 trial | Began end of May 2021. |
| CoVLP Medicago Canada |
30,918 | 1 trial | Began in March 2021. |
| CVnCoV CureVac Germany |
41,140 | 1 large, several small | Large international trial fully recruited. |
| EpiVacCorona VECTOR Russia |
3,000 | 1 trial | Apparently fully recruited. |
| GrAd-CoV-2 ReiThera Italy |
10,300 | 1 trial | Phase 2/3 began February/March. |
| mRNA-1273 Moderna USA (NIH) |
40,170 | 1 large, 3 small |
Trial of 30,420 reported. Trial of 3,000 adolescents reached full recruitment in March. (Trial for booster modified for variant began in March, and so did a trial in infants and children.) |
| Nanocovax Nanogen Vietnam |
13,000 | 1 trial | Began in June 2021. |
| NVX-CoV2373 Novavax USA |
46,600 | 3 trials |
Trial of 15,000 in UK fully recruited – results published. US/Mexico trial of 30,000 fully recruited – press release readout. (Efficacy results also from 4,442 in a phase 2b trial in South Africa.) Small bridging trial in India began in March. |
| SCB-2019 Clover/ Dynavax China/USA |
22,000 | 1 trial | Trial began in March. |
| SOBERANA-2 Finlay Institute Cuba |
44,010 | 1 trial | Began in March; fully recruited in Cuba (efficacy briefly reported) and Iran. |
| Sputnik V, Sputnik Light (Gam-COVID-Vac) Gamaleya Russia |
41,700 | Mostly single trial. Small trial of Sputnik Light. |
Trial in Russia stopped recruiting at 31,000 – results published. Small trial in UAE fully recruited; small trial in India has completed dosing. Sputnik Light trial began in February. |
| ZF2001 Chinese Academy of Sciences |
29,000 | Single trial | Started in November. |
| ZyCov-D5 Zydus Cadila India |
30,000 | Single trial | Fully recruited. |
| Inactivated virus vaccines | |||
| BBIBP & WIBP Sinopharm China |
60,300+ | 2 large trials, several small |
Trial with 45,000 fully recruited – results published. Trial with 12,000 fully recruited in Peru. |
| CoronaVac Sinovac China |
31,020+ | 2 large trials, several small, 1 unknown |
Trial with 13,060 healthcare workers in Brazil fully recruited – results published. |
| Covaxin Bharat Biotech, Indian Medical Research Council India |
~34,500 | Mostly a single trial | Trial efficacy readout for 25,800 participants. |
| COVIRAN BAREKAT Shifa-Pharmed Iran |
20,000 | Single trial | Started in April. |
| KCONVAC Shenzhen Kangtai Bio China |
28,000 | Single trial | Started in June. |
| QazVac Scientific Research Institute Kazakhstan |
3,000 | Single trial | Fully recruited, results expected in April. |
| TurkoVac (Erucov-Vac) Health Institutes of Turkey/TC Erciyes Uni |
40,800 | Single trial | Started in June. |
| (Unnamed) IMBCAMS, Chinese Academy of Sciences |
34,020 | 1 trial | Began in January. |
|
VLA2001 |
4,000 | Single trial | Superiority immunogenicity trial, VLA2001 vs ChadOx1. Started in April. |
| Total participants |
Over 940,000 |
About 600,000 people likely to be already recruited. |
The phase 3 trials, and the preclinical and clinical trial results reported for those vaccines
| Vaccine Company Country |
Preclinical | Phase 1 results People |
Phase 1/2 results People |
Phase 2 results People |
Phase 3 Total people |
| Abdala CIBG/ BioCubaFarma Cuba |
Unpublished | n.a. |
Unpublished (132) |
n.a. |
48,000 (Cuba) (Trial planned in Venezuela) |
| Ad26.COV2-S Johnson & Johnson USA |
Primate, non-primate | n.a. | 1,045 |
n.a. (Trial for 824 pregnant woman to start) |
73,783: 43,783 (single shot) (Argentina, Brazil, Chile, Colombia, Mexico, Peru, South Africa, USA) Results reported 30,000 (2 shots) (Belgium, Colombia, France, Germany, Philippines, South Africa, Spain, UK, USA) |
| Ad5-nCoV CanSino China |
Non-primate only | 108 | n.a. |
508 (Plus unreported trial in children) |
40,500: 40,000 500 |
| ArcoV Walvax/Chinese Academy of Military Science |
Non-primate only | Unpublished (168) | n.a. |
Unpublished (420) |
28,000 (Mexico) |
| BECOV2 (Corbevax) Biological E India |
Non-primate only | n.a. | Unpublished (360) | n.a. | (Seeking approval in India) |
| BNT162b1-b3 BioNTech/Pfizer Germany |
Primate, non-primate (b2 only) | b1:
45 b1: 60 b1 & b2: 195 |
n.a. |
(Phase 2/3 trial) (Phase 2 for people with allergies underway: 3,400, includes Moderna) (Bridging trials in China & Japan) |
51,945 46,331 (b2)* 4,000 (b2)** (USA) (pregnancy) 1,530 (b2) (USA) (consistency) 84 (lyophilized b2) (USA) |
| ChAdOx1 nCov-19/AZD1222 AstraZeneca UK (Oxford Uni) |
Primate, non-primate | n.a. | 1,077 |
560 (from the major phase 2/3 trial) 2,026 (from South Africa, HIV-negative) 300 (Phase 2 trial underway for ages 6 to 17) |
54,690: 12,390** 30,000 10,300 1,600** 400 –100***– |
|
CoV2 preS dTM |
Unpublished | n.a. | 441 | n.a. | |
|
CoVLP |
Non-human primates | 180 | n.a. | 588 | |
|
CVnCoV |
Primate, non-primate | 247 | n.a. | (Phase 2/3 trial) |
41,400 36,500** 2,520 1,000 (with flu vax) (Argentina, Colombia, Peru) 180 (comparing older people to younger) (Not stated) 1,200 (people with co-morbidities) (Not stated) |
|
EpiVacCorona |
Unpublished | 14 | n.a. | 86 |
3,000 |
|
GrAd-CoV-2 |
Primate, non-primate | 90 | n.a. | (Phase 2/3 trial) |
10,300** |
| mRNA-1273 Moderna/NIH USA |
Primate, non-primate |
85 (Phase 1 for variant modification underway: 210) |
n.a. |
600 (Phase 2 for people with allergies underway: 3,400, includes BNT-Pfizer) |
40,170 30,420 adults 3,000 young people** 6,750 infants and children** |
|
Nanocovax |
Unpublished | n.a. | Unpublished (620) | n.a. |
13,000 |
| NVX-CoV2373 Novavax USA |
Primate, non-primate | 131 | n.a. |
1,288 (Australia, USA) 4,387 (South Africa) |
45,000: 15,000 30,000 Small bridging trial planned in India |
| SCB-2019 Clover/Dynavax China/USA |
Primate, non-primate | 148 | n.a. |
(Phase 2/3 trial) |
22,000** (Brazil, Philippines – South African & European sites planned) |
|
SOBERANA-2 (Finlay-Fr-2)/SOBERANA-1 (Finlay-Fr-1) |
2: Non-primate 1: Unpublished |
2: Unpublished (40) 1: 30 (Unpublished studies for 676 & 60) |
n.a. |
2: Unpublished (910) |
44,010 |
|
Sputnik V & Sputnik Light (Gam-COVID-Vac) |
Unpublished | n.a. |
76 Sputnik Light: (Phase 1/2 trial underway: 110) |
n.a. |
41,700 31,000 100 2,000 1,600** 1,000 6,000 – Sputnik Light (Russia) |
|
ZF2001 |
Primate, non-primate |
50 (Unpublished, 50 aged 60+) |
n.a. | 900 |
29,000 |
|
ZyCov-D |
Primate, non-primate | n.a. | Unpublished (1,048) | n.a. |
30,000 |
| Inactivated vaccines: | |||||
| BBIBP & WIBP (Beijing & Wuhan) Sinopharm China |
Primate, non-primate (Wuhan) Unpublished |
n.a. | 320 (Wuhan)
640 |
n.a. |
60,300+: 45,000 (both vaccines) 3,000 (Beijing only) 300 (Wuhan only) Apparently additional: 12,000 (both vaccines) (originally 6,000) ? (both vaccines) ? (Beijing only) |
| CoronaVac (PicoVacc) Sinovac China |
Primate, non-primate | 239 | 572 (aged 3 to 17) | 950 |
31,020+: 13,060 (originally 9,000) 13,000 1,620 Unknown – for people 60+ 2,300 1,040 (non-inferiority study of vaccine lots) |
| Covaxin (BBV152) Bharat Biotech/Indian Medical Research Council India |
Non-primate | 375 | n.a. | 380 |
~34,500: 28,500 (India) Efficacy readout in press release 525** (India, aged 2 to 17) 1,000-2,000 (planned, Bangladesh) 4,500 (planned, Brazil) |
| COVIRAN BAREKAT Shifa-Pharmed Iran |
Primate, non-primate | Unpublished (88) | n.a. | n.a. (Phase 2/3) |
20,000** (Iran) |
| KCONVAC Shenzhen Kangtai Bio (China) |
Unpublished | 60 | n.a. | 500 |
28,000 (Malaysia, reportedly also planned for Argentina, Colombia, Pakistan, Philippines, Ukraine) |
| Qaz-Covid-in Scientific Research Institute Kazakhstan |
Unpublished | n.a. | Unpublished (244) | n.a. |
3,000 (Kazakhstan) |
| TurkoVac (Erucov-Vac) Health Institutes of Turkey/TC Erciyes Uni |
Unpublished | Unpublished (44) | n.a. | Unpublished (250) |
40,800 (Azerbaijan, Hungary, Poland, Turkey, Uzbekistan) (Equivalence trial with Sinovac CoronaVac) |
| Unnamed IMBCAMS/Chinese Academy of Medical Sciences |
Non-primate | 191 (aged 18 to 59) | Unpublished: 471 (aged 60+) | 742 (aged 18 to 59) |
34,020 (Bangladesh, Brazil, Malaysia) |
| VLA2001 Valneva France |
Unpublished | n.a. | Press release only (153) | n.a. |
4,000 (UK) (VLA2001 vs ChadOx1) |
* a combined phase 1/2/3 trial
** a combined phase 2/3 trial
*** an unrandomized phase 3 trial
n.a. = not applicable
Sources: unless otherwise linked, the sources are from my tagged public Zotero collection (detailed below)
~~~~

All my Absolutely Maybe Covid-19 vaccine posts
All previous Covid-19 posts at Absolutely Maybe
My posts at The Atlantic, at WIRED, and debunking posts at my personal website.
Disclosures: My interest in Covid-19 vaccine trials is as a person worried about the virus, as one of my sons is immunocompromised: I have no financial or professional interest in the vaccines. I have worked for an institute of the NIH in the past, but not the one working on vaccines (NIAID). More about me.
The cartoons and infographics are my own (CC BY-NC-ND license). (More cartoons at Statistically Funny.)
Infographic notes:
The infographic scale was determined by 95% as the highest established efficacy rate with 50% as the minimum set by WHO and the US FDA, enabling a breakdown into equal-sized low-medium-high areas: data shown in table. The low-medium-high scale for rates of adverse events was set with the rate for the inactivated vaccines as lowest established rate, with the highest rate just above the highest for the most common systemic adverse event any of these vaccines at the other end (75%), after taking both the rate of the most commonly reported severe outcome and the age of the trial population into account.
Sources for study records
Source records are in my public Zotero collection of Covid-19 vaccines that have any published preclinical or clinical results (or preprints), or that are in phase 3 trial (more details below, including how to use it) for general populations. It also includes the matched control studies I am featuring. Please let me know if I’m missing any! On July 1, the collection included 715 entries:
- 168 vaccines/groups with published or posted results;
- 27 trial protocols, for 10 vaccines and Com-COV (including 1 vaccine with additional protocol versions in a clinical study report package);
- 215 preclinical preprints/articles;
- 82 preclinical or serum studies of vaccines and new variants;
- 218 trial registry entries associated with these vaccines;*
- 81 clinical trial preprints*/articles/letters:
- 27 for phase 1 trials;
- 18 for phase 1/2 trials;
- 13 for phase 2 trials;
- 2 combined reports of phase 2 and 3 trials;
- 1 combined report of phase 1 to 3 trials;
- 11 reports from phase 3 trials (for 8 vaccines);
- 2 reports on boosters;
- 4 author replies to letters to the editor about their publications (for 3 vaccines);
- 3 errata notices for 2 vaccines;
- 34 trial efficacy readouts (press releases or website), for 17 vaccines;
- 29 documents reporting on or reviewing phase 3 data at regulatory agencies, for 8 vaccines;
- 2 full clinical study report packages;
- 4 termination notices, for 5 vaccines (all but one of which have other records in this collection);
- 24 reports of 23 featured community impact studies, for 5 vaccines;
- Records for 9 vaccines tagged as trials including children/teenagers;
- Records for 4 vaccines tagged as adapted for variant;
- Record for 1 vaccine tagged as geographic.
* May not include all entries where a trial is registered in multiple registers, a preprint is posted to multiple servers. There can be multiple records for one study if I happened to pick up that a published version is substantially different to a preprint.
Notes on the collection
This is a publicly accessible collection I update regularly. It includes any Covid-19 vaccine with published preclinical, clinical trial results, or trial protocols. If a phase 3 trial starts (or is about to) without any prior publications, that trial would also be included. Once a vaccine is in the collection, clinical trial register entries for that vaccine are also added.
When trials are registered in more than clinical trials registry, the multiple records may or may not be in the collection: If I have located a record in ClinicalTrials.gov, I do not hunt for additional registrations. For my own convenience in keeping an overview of vaccine progress, when preprints appear later in journals, I over-write the original record with the journal article. Preprints may also be uploaded to multiple preprint servers: rather than check if versions have differed, I keep the first preprint in the collection (sometimes over-written with updates in the same server), unless I saw that a version that seems markedly different.